NMR Theory - PowerPoint PPT Presentation
Title:
NMR Theory
Description:
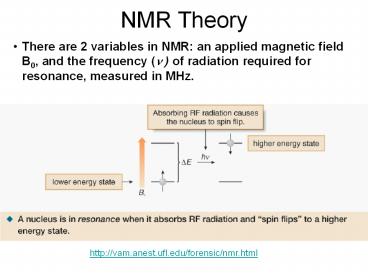
NMR Theory There are 2 variables in NMR: an applied magnetic field B0, and the frequency ( ) of radiation required for resonance, measured in MHz. – PowerPoint PPT presentation
Number of Views:116
Avg rating:3.0/5.0
Title: NMR Theory
1
NMR Theory
- There are 2 variables in NMR an applied magnetic
field B0, and the frequency (? ) of radiation
required for resonance, measured in MHz.
http//vam.anest.ufl.edu/forensic/nmr.html
2
Effect of B0 on resonance frequency
- NMR spectrometers are designated according to the
frequency required to make protons resonate. The
modern standard is 300 MHz. However,
manufacturers are actively pursuing stronger
magnets. 900 MHz is currently as high as it gets.
3
Schematic of an NMR
4
Resonance Frequency
- Different nuclei resonate at greatly different ?
on a 300 MHz instrument (1H 300 MHz) 13C
resonates at 75 MHz. - The same type of nucleus also absorbs at slightly
different ?, depending on its chemical
environment. - Exact frequency of resonance chemical shift
- The strength of the magnetic field actually felt
by a nucleus (Beff) determines its resonance
frequency. - Electron clouds shield the nucleus from the
magnet - Circulation of electrons in p orbitals can
generate local magnetic fields that influence
Beff - Modern NMR spectrometers use a constant magnetic
field strength B0, and pulse a broad range of
frequencies to bring about the resonance of all
nuclei at the same time.
5
Chemical Shift
- Peaks on NMR spectrum resonances .
- Chemical shift is measured in ppm
- ppm ? in Hz relative to ref peak/instrument ?
in MHz. - Protons absorb between 0-10 ppm. C-13 nuclei
absorb between 0-250 ppm. - Reference peak 0 ppm (CH)4Si
tetramethylsilane (TMS). TMS is an inert compound
that gives a single peak at higher frequency than
most typical NMR peaks.
6
Electronic Shielding
7
Shielding in Spectrum
8
1H NMR Interpretation
- Number of Resonances
- Chemical Shifts
- Integrations
- Splitting Patterns
- Exchangeable Protons
9
Number of Resonances
10
Stereochemistry
- Watch out when you have rings and/or double
bonds! To determine equivalent protons in
cycloalkanes and alkenes, always draw all bonds
to hydrogen.
11
Number of Signals in a Cyclic Compound
- Proton equivalency in cycloalkanes can be
determined similarly.
Types of NMR relationships 1. chemically
equivalent 2. coincidentally equivalent 3.
non-equivalent, enantiotopic 4. non-equivalent,
diastereopic 5. non-equivalent. Use substitution
criterion to decide.
12
Chemical Shift - Local Diamagnetic Shielding
13
Induced Anisotropic Shielding - Benzene
- In a magnetic field, the six ? electrons in a
benzene ring circulate around the ring creating a
ring current. - The magnetic field induced by these moving
electrons reinforces the applied magnetic field
in the vicinity of the protons. - The protons feel a stronger magnetic field and
thus are deshielded. A higher frequency is needed
for resonance.
14
Induced Anisotropic Shielding - Alkenes
- In a magnetic field, the loosely held ? electrons
circulate creating a magnetic field that
reinforces the applied field in the vicinity of
the protons. - Since the protons now feel a stronger magnetic
field, they require a higher frequency for
resonance. Thus the protons are deshielded and
the absorption is downfield.
15
Induced Anisotropic Shielding - Alkyne
- In a magnetic field, the ? electrons of a
carbon-carbon triple bond are induced to
circulate, but in this case the induced magnetic
field opposes the applied magnetic field (B0). - Thus, the proton feels a weaker magnetic field,
so a lower frequency is needed for resonance. The
nucleus is shielded and the absorption is upfield.
16
Summary of pi electron effects
17
Characteristic Shifts
18
Integrations
- The integration of each resonance is proportional
to the number of absorbing protons. - The integral ratios tell us the ratios of the
protons causing the peak. - Strategy - find a peak that you can assign
unambiguously and set its integral at the
appropriate number of Hs.
19
Splitting Patterns
- Consider the spectrum below
20
Theory of spin-spin splitting
- Spin-spin splitting occurs only between
nonequivalent protons on the same carbon or
adjacent carbons.
Let us consider how the doublet due to the CH2
group on BrCH2CHBr2 occurs
21
Triplet
Let us now consider how a triplet arises
- When placed in an applied magnetic field (B0),
the adjacent protons Ha and Hb can each be
aligned with (?) or against (?) B0. - Thus, the absorbing proton feels three slightly
different magnetic fieldsone slightly larger
than B0, one slightly smaller than B0, and one
the same strength at B0.
22
Triplet
23
Peak ratios in a multiplet.
- Doublet The two spin states of the proton
causing splitting are nearly equally populated
(because the energy difference is so small).
Therefore a doublet is has a peak ratio of 11. - Triplet - Because there are two different ways
to align one proton with B0, and one proton
against B0that is, ?a?b and ?a?bthe middle peak
of the triplet is twice as intense as the two
outer peaks, making the ratio of the areas under
the three peaks 121. - Higher use Pascals triangle
24
Multiplet names
25
Rules for predicting splitting patterns
- Equivalent protons do not split each others
signals. - A set of n nonequivalent protons splits the
signal of a nearby proton into n 1 peaks. - Splitting is observed for nonequivalent protons
on the same carbon or adjacent carbons.
If Ha and Hb are not equivalent, splitting is
observed when
26
(No Transcript)
27
Nuclear Magnetic Resonance Spectroscopy
1H NMRSpin-Spin Splitting
Whenever two (or three) different sets of
adjacent protons are equivalent to each other,
use the n 1 rule to determine the splitting
pattern.