Conductivity Lab Report Due - PowerPoint PPT Presentation
1 / 24
Title:
Conductivity Lab Report Due
Description:
Organic substances were originally though to possess a 'vital force' which made ... Two chiral molecules. optical isomers of 3-methylhexane. optical isomers of alanine ... – PowerPoint PPT presentation
Number of Views:55
Avg rating:3.0/5.0
Title: Conductivity Lab Report Due
1
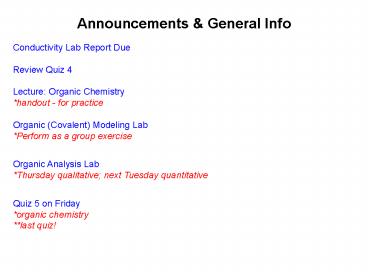
Announcements General Info
Conductivity Lab Report Due Review Quiz
4 Lecture Organic Chemistry handout - for
practice Organic (Covalent) Modeling Lab
Perform as a group exercise Organic Analysis
Lab Thursday qualitative next Tuesday
quantitative Quiz 5 on Friday organic
chemistry last quiz!
2
Organic Chemistry
- Organic substances were originally though to
possess a vital force which made them different
from inorganic compounds. - 1828 Wöhler converted ammonium cyanate
(inorganic) to urea (organic), showing that
organic compounds were subject to the same laws
as any other chemicals. - Organic chemistry is the chemistry of carbon,
which has the ability to bond to itself to form
chains and rings. - Organic compounds consist of a backbone of
carbon atoms, with hydrogens to complete the
valence. - Other atoms form functional groups which is
what distinguishes different classes of organic
molecules.
Old definition Organic compounds are substance
originating from organisms
Current definition Organic compounds are
carbon-based compounds.
3
Bonding in Organic Compounds (Covalent
Bonds!!!) Hydrocarbons are organic compounds
which contain only carbon and hydrogen Drawing
organic compounds From the electron-dot formula
to the expanded structural formula Expanded
structural formula is written when the bonds
between all of the atoms are shown C
4H ? H C H H C H
H
H
H
H
Condensed structural formula is written when each
carbon atom and its attached hydrogen atoms are
depicted as groups H C CH3 C
CH2
H
H
H
H
Expanded Condensed Expanded
Condensed
4
The Tetrahedral Structure of Carbon The VSEPR
theory still applies here! Lets look at the
three-dimensional structures of methane and
ethane
Still have tetrahedral shapes (just add them
together!) All angles are 109.5º with methane,
and close to 109.5º with ethane
5
Alkanes - General formula CnH2n2 Alkanes are a
class (family) of organic compounds that contain
only carbon and hydrogen atoms and only single
bonds. Alkanes are saturated (NO double
bonds). They are connected in a row or a
continuous chain and follow the IUPAC system
International Union of Pure and Applied
Chemistry (IUPAC) system is used to name organic
compounds Take the prefix and add ane to the
end to form the name!
Memorize!!!
6
Table 15.1 Numerical Roots for Carbon Chains and
Branches
Number of C atoms
Roots
meth-
1
eth-
2
prop-
3
but-
4
pent-
5
hex-
6
hept-
7
oct-
8
non-
9
dec-
10
- Add ane to root to get name of alkane.
7
Alkanes From the previous slide, we know that
continuous means connected in a row. If a chain
consists of three carbon atoms or more, the
carbon atoms do NOT lie in a straight line Lets
look at hexane The covalent bonds in are
not in fixed positions. They are free to rotate!!
Same molecule!!!
8
Alkanes
Remember to count up all your carbon and hydrogen
atoms for the molecular formula
Theres more than one way to draw the condensed
structural formula because of free rotation
9
Sample Problem Drawing Expanded and Condensed
Structural Formulas for Alkanes A molecule of
butane, C4H10 has four carbon atoms in a row.
What are its expanded and condensed structural
formulas?
10
Cycloalkanes are cyclic hydrocarbons. They form
rings! Nomenclature A cycloalkane is named by
adding the prefix cyclo to the name of the alkane
with the same number of carbon atoms
11
- Sample Problem
- Naming Alkanes
- Give the IUPAC name for each of the following
- CH3 CH2 CH2 CH2 CH3
- D
- CH3 CH2 CH3
12
Skeletal Isomers When a hydrocarbon is composed
of four or more carbon atoms, it can have side
groups attached to the main carbon chain. These
side groups are referred to as branches or
substituents. A branched alkane contains at
least one branch A skeletal isomer is an organic
compound in which identical molecular formulas
have different arrangements of carbon atoms (I.e.
different branching)
13
SKELETAL ISOMERS OF C5
14
Sample Problem Identify each pair of structural
formulas as skeletal isomers or the same
molecule a. b.
15
Positional Isomers
- Alkanes can be halogenated to give haloalkanes
- Two isomers of chloropropane (C3H7Cl)
1-chloropropane
2-chloropropane
- These are positional isomers The same carbon
skeleton with different positions of the
functional group. - Note 3-chloropropane is the same as
1-chloropropane
16
Functional Isomers A functional isomer is an
isomer with structural differences that place
them in different classes of organic compounds.
They have the same molecular formula, but have
either different functional groups or different
degrees of unsaturation.
CH3CH2OH and CH3OCH3
-
-
CH3CH2CH2C CCH3 and CH3CHCH-CHCHCH3
-
17
Alkenes and Alkynes Unsaturated Hydrocarbons are
compounds composed of carbon and hydrogen in
which the carbon chain contains at least one
double or triple carbon-carbon bond
An alkene contains at least one double
carbon-carbon bond General formula CnH2n
In alkenes, there is NO rotation around the
carbons in the double bond. It is rigid
(fixed!). An alkyne contains at least one
triple carbon-carbon bond General formula
CnH2n-2 Triply bonded carbons can only have
one other bond, so geometry is linear.
18
Cis-Trans Isomers - for double bonds! In
alkenes, there is NO rotation around the carbons
in the double bond. It is rigid (fixed!). In a
cis isomer, two similar groups are on the same
side of the double bond In a trans isomer, the
similar groups are on opposite sides of the
double bond In general, trans isomers are more
stable than cis isomers because the large groups
are further apart Not every alkene shows
cis-trans isomerism Ex If a carbon from the
double bond has identical groups on
it Nomenclature - place a cis or trans in front
of the name followed by a dash
19
Sample Problem Identifying Cis-Trans
Isomers Identify the following as cis or trans
isomers
20
Geometric Isomers A geometric isomer (also
known as cis-trans isomers) is an isomer with
different orientations of groups around a double
bond (or similar structural feature).
21
Stereoisomers (optical isomers)
- Since carbon is tetrahedral, these molecules are
really the same (you can build a model to prove
this). - If all four substituents on C are different,
however, two stereoisomers (optical isomers) are
obtained. The molecules are chiral.
22
Figure 15.8
An analogy for optical isomers
23
Two chiral molecules
Figure 15.9
24
The Binding Site of an Enzyme
Figure 15.11