Combustion Fire Fundamentals - PowerPoint PPT Presentation
1 / 25
Title: Combustion Fire Fundamentals
1
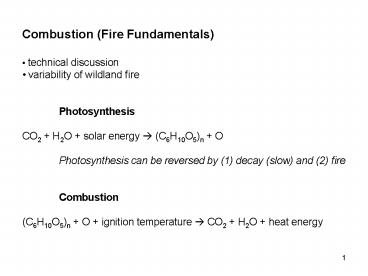
- Combustion (Fire Fundamentals)
- technical discussion
- variability of wildland fire
- Photosynthesis
- CO2 H2O solar energy ? (C6H10O5)n O
- Photosynthesis can be reversed by (1) decay
(slow) and (2) fire - Combustion
- (C6H10O5)n O ignition temperature ? CO2 H2O
heat energy
2
Fire fundamentals triangle oxygen, heat, fuel
Ontario Aviation and Forest Fire Management
3
- Heat heat transfer
- Heat
- Heat is a form of energy, thermal energy,
energy of molecular motion - Temperature is a function of the motion of
molecules, measured in degrees - Heat of preignition is heat required to raise
fuel to ignition temperature, usually 600o F
(300o C). Note that heat of preignition is
therefore related to fuel temperature. - Heat of combustion is heat required to maintain
chain reaction of combustion ( heat value or
heat content). Usually 8000 Btu/lb (range of
forest fuels 7400-9400 Btu/lb). - Note English vs. metric units.
4
Heat flux Amount of heat per unit fuel per unit
time, or energy per unit area. Example
Btu/lb/s or KJ/kg/s Also heat release rate or
intensity Not a property of the fuel ? same fuel
can burn more or less intensely, depending on
moisture content, aeration, temperature.
Example cool smoldering fire vs. hot flaming
fire
5
Heat transfer is the movement of heat energy,
occurs whenever there is a heat difference
between media. Example fire is hot, so heat is
transferred to surrounding soil, air,
fuels. What are ways in which heat is
transferred?
6
Conduction is heat transfer by molecular
activity. Example sun heats earth's surface,
warmth is conducted through the soil.
Different substances conduct differently
scale from air (poor conductor), water, wood,
metal like copper (excellent conductor). What
will conduct better, high-density or low-density
material?
7
Dense materials conduct better because molecules
are closer together. A dense material has
higher heat capacitymore heat needed to ignite
itand conducts heat better into the interior so
the surface heats up more slowly. Example this
is one reason why solid wood (dense) requires a
lot of heat to ignite, but rotten wood can be
ignited with a spark. Conduction is the primary
mode of heat transfer within fuel materials like
logs.
8
Convection is heat transfer by movement of liquid
or gas. Hot air currents from fires heat
higher vegetation (shrubs, canopy)and heat
firefighters. Convection is the primary mode
of heat transfer to higher vegetation in wildland
fires.
9
Radiation is heat transfer by electromagnetic
energy to objects capable of absorbing it.
Radiation travels in straight lines, so
intensity depends on the angle of the absorbing
object (perpendicular most intense). This will
be important when we look at fuel arrangements
(fuel ladders) and the effect of slopes (fuels
tilted above or below the heat source).
Radiation drops off with the square of the
distance from the source, so twice the distance
one-fourth the intensity. Heat transfer
equations presented on pp. 13-14.
10
Phases of combustion Preignition Dehydration Pyro
lysis Ignition Flaming combustion Smoldering
combustion Extinction
11
Preignition raises temperature of the fuel,
evaporating water and volatilizing compounds
(changing them to gases). Preignition is
endothermic (energy is absorbed by the fuel to
heat it) combustion is exothermic (releases heat
energy) ignition is the transition point where
the reaction becomes self-sustaining.
12
Dehydration removes volatiles, water others.
Some volatiles begin to come off at low
temperatures (Example pine smell). Water is a
key volatile fuel moisture can range from lt 10
to gt 300 and water requires high heat to change
from liquid to gas. Fuel temperatures won't rise
above 212o F until water is driven off. Recall
that ignition temperature is around 600o F, so
fire won't continue if the heat produced is
insufficient to dry and preheat a moist fuel.
13
(No Transcript)
14
Pyrolysis is thermal degradationbreakdownof
plant chemicals due to heat, producing volatiles
for combustion. Different pyrolysis pathways
are shown in Figure 1.7, leading to different
forms of combustion (glowing, flaming). Key
point is that the great variety of different
chemicals in plants will decompose in various
ways, leading to distinct fire behavior and
emissions. Plants with many volatile chemicals,
like oils waxes, may burn more intensely
(pines, chaparral contrast hardwood litter).
15
Ignition "process by which a rapid, exothermic
reaction is initiated, which then propagates"
(Pyne et al. 1996, p. 18). A fire is a series of
ignitions, following the sequence of preheating,
dehydration, and pyrolysis. Lightning is the
dominant cause of forest fires on earth 8
million strikes per day, temperature millions of
degrees. However, fire occurrence depends on the
fuel and weather factors of the strike study
cited in text showed 0.1-1 of strikes started
fires. (Lightning kills 150, injures 250 per year
in US Peterson's Guide Atmosphere). Spontaneous
combustion can occur under specific conditions,
outlined in Pyne et al. 1996, pp. 19-20 Other
ignition sources include humans (to be discussed
extensively later), volcanoes, monkeys throwing
rocks (South Africa).
16
Flaming combustion is rapid oxidation of volatile
gases produced by pryolysis (heat decomposition
of fuel). Gases must be mixed with oxygen.
Usually combustion is limited by oxygen because
fuels are compactly arranged, so wind has a major
effect on flaming combustion. Flame
temperatures average 1300-1800o F, maximum might
be 2200-3000o F. Pyrolized particulates can burn
in the flame (giving it the yellow color).
Unburned particulates form smoke.
17
(No Transcript)
18
Smoldering/glowing combustion includes the
pyrolysis zone, the glowing charred zone (maximum
temperature), and the residual char ash.
Fires usually smolder in tightly packed fuels
(duff) or those with a chemical content
(pyrolysis pathway) more conducive to smoldering
(Example decomposing material with high lignin
content). Smoldering fires burn slowly (1
inch/hr), so they heat the soil and plants
longer. Soil temperatures can reach 500-1100o F,
lower than flaming temperatures but for a much
longer period. Volatiles emitted can be quite
different from those consumed in flaming
combustion, including tars and high-boiling point
liquids that can form aerosols. Key point is
smoldering fires can cause more heat and more
smokeand dirtier smokethan flaming fires.
19
Extinction occurs when preheating is insufficient
to drive off moisture, or fuels become less
volatilizable, for instance by having a high
inorganic content (inorganic minerals absorb heat
but never give off volatile compounds). Thus two
common firefighting strategies are to add water
or to mix fuels with inorganic soil. Moisture
of extinction varies with fuel types grass and
litter stop burning around 20 moisture content,
but live mature chaparral (100) can burn.
20
Combustion products are very important because
air quality is central to fire management. Smoke
has immediate effects (firefighter health,
visibility), regional effects (haze, respiratory
problems), and global effects (climate change,
greenhouse gases). H2O and CO2 are given off in
combustion (reverse of photosynthesis). Combustio
n efficiency is the ratio of actual CO2 to
maximum possible CO2. Usually ranges 50-95.
Usually highest for flaming fires. Particulate
emissions are inversely related to efficiency.
21
Other carbon compounds CO (carbon monoxide) is
highly hazardous. PM2.5 (small particulates less
than 2.5 microns diameter) are respirable (health
hazard because they can be drawn into the lungs).
CH4 (methane) is a greenhouse gas like CO2.
Other emissions include oxides of nitrogen,
sulfer (essential plant nutrients and, in the
atmosphere, pollutants), and other minerals.
Key point smoke quantity and composition is
related to the fuel being consumed and the type
of combustion. Smoldering fires produce more
particulates.
22
Fuels Intrinsic properties of fuels are inherent
characteristics, like chemistry and density. We
will look later at the extrinsic properties of
fuels, such as abundance and arrangement.
Plants consist of organic compounds, about 50 C,
44 O, 5 H by weight. Cellulose is a sugar
which forms linear structures (microfibrils)
giving structural strength rigidity to cell
walls. Cellulose pyrolizes rapidly, leading to
flaming combustion. Hemicelluloses are
carbohydrates (polysaccharides) structurally
similar to cellulose. Lignin is an aromatic
polymer which gives wood its stiffness. Lignin
is more resistant than cellulose to decay, so the
ratio of lignin increases as wood decays from
16-33 to as much as 65. Lignin pyrolizes
poorly and leads to smoldering combustion,
therefore also leading to dirtier
smoke. Extractives are various other compounds
(alcohols, hydrocarbons, waxes, oils) which
volatilize and affect fire behavior
(flammability).
23
The large lignin molecules fill three dimensions
and are heavily cross linked. Sometimes lignin is
isolated as a brown powder, but more often it is
a gummy mixture of lignins with a wide range of
molecular weights. Lignin resists attack by most
microorganisms, and anaerobic processes tend not
to attack the aromatic rings at all. Aerobic
breakdown of lignin is slow and may take many
days. Lignin is nature's cement along with
hemicellulose to exploit the strength of
cellulose while conferring flexibility.
Lignin
http//www.eng.rpi.edu/dept/chem-eng/Biotech-Envir
on/FUNDAMNT/lignin.htm
Cellulose
http//www.psrc.usm.edu/macrog/cell.htm
24
Fuel consumption is usually far less than 100.
Available fuel is the amount of fuel which be
burned by a given fire, usually much less than
the total aboveground biomass on the site.
Consumption is related to the amount of heat
produced. An example of consumption in Figure
1.15 (p. 30) shows about 50 consumption of woody
fuels. Duff consumption can be expressed in
terms of depth (or percentage of depth)
reduction, or percentage of mineral soil exposed.
(Depth of the forest floor is much easier to
estimate than mass, as we'll discuss later).
25
Example of lighting a match. What is required
for fire? Fuel (matchstick), oxygen, and
ignition temperature, created by friction on the
box. What happens as the match burns? Heat is
conducted through the wood radiated ahead of
the flame, heat smoke rise in a convection
column. The wood is preheated, driving off water
volatile chemicals and pyrolizing the fuel.
Volatile gases mix with oxygen and oxidize in the
flame (suspended above the match head). Glowing
combustion continues after the flaming front
passes, evolving relatively more smoke.