Chapter 13: Solutions and Their Properties - PowerPoint PPT Presentation
1 / 71
Title:
Chapter 13: Solutions and Their Properties
Description:
Solvation of the solvent, A ... strong solution - as in a strong cup of tea ... Caffeine (C8H10N4O2) is a stimulant found in coffee and tea. ... – PowerPoint PPT presentation
Number of Views:314
Avg rating:3.0/5.0
Title: Chapter 13: Solutions and Their Properties
1
Chapter 13 Solutions and Their Properties
2
Chapter 13 Outline
- The Solution Process
- Energy changes
- Saturated Solutions and Solubility
- Factors Affecting Solubility
- Ways of Expressing Concentration
- Colligative Properties
- Vapor Pressure Lowering, BP Elevation, FP
Depression, Osmosis - Fractional Distillation of Liquid Mixtures
3
Solutions
- Solution
- mixture of two or more substances
- components are the solute and the solvent
- Solvent
- component of the mixture present in greater
quantity - often retains its physical characteristics
- Solute
- component of the mixture present in smaller
quantity
4
Solution Process
- Intermolecular Forces
- must be overcome in both the solvent solute
before the mixture can be formed - forces between the solute solvent must be
similar in nature and strength - hydration
- interaction between water (the solvent) solute
is termed hydration - solvation
- interaction between any solvent the solute
5
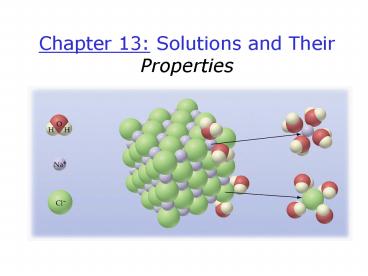
Ion-Dipole
6
Hydration of NaCl
7
05_09.jpg
A
8
Energy Changes And Solution Formation
NaCl dissolves in water because water has
sufficient attraction for the Na and Cl- ions to
overcome the attraction for one another in the
crystal
9
Energy Relationships
- DHsolution DH1 DH2 DH3
- DH1 energy required to separate solute
particles - DH2 energy required to separate solvent
particles - DH3 energy produced by interaction of
solute/solvent - DHsolution is exothermic only if
- DH3 is larger than DH1 DH2
10
(No Transcript)
11
Solubility
- General Rule -- like dissolves like
- polar substances will dissolve other polar
substances - non-polar substances will dissolve other
non-polar substances - polar substances will not dissolve non-polar
substances
12
Solubility
Processes in which the order of the system
increases tend to occur spontaneously
13
Solubility
- Polar/Polar solutions
- driven by energy relationships -- DH3 is larger
than DH1 DH2 - Non-polar/Non-polar solutions
- driven by disorder -- DH3 is about the same as
DH1 DH2
I2 in hexane
14
(No Transcript)
15
Cell Membrane
16
Conceptual Question
- The dissolution of water in octane (C8H18) is
prevented by__________? - London dispersion forces between octane molecules
- Hydrogen bonding between water molecules
- Dipole-dipole attraction between octane molecules
- Ion-dipole attraction between water and octane
molecules - Repulsion between like-charged water and octane
molecules
17
Conceptual Question
- When two nonpolar organic liquids are mixed, a
solution forms and the enthalpy of solution is
quite small. Label the two organic liquids as A
(solvent) and B (solute). The formation of
solution is favored by__________ - Hydration of the solute, B
- The equal enthalpy of the solvent and solute
- The highly negative enthalpy of the solution
process - Solvation of the solvent, A
- An increase in disorder, since A-A, B-B, and A-B
interactions are similar
18
Saturated Solutions Solubility
- Process
- solute solvent solution
- Solubility
- quantity of solute necessary to produce a
saturated solution - generally in grams of solute per 100 grams or mL
of water
Solution formation
crystallization
19
Saturated Solutions Solubility
- Saturated Solution
- solution is in equilibrium with undissolved
solute - solution contains the maximum amount of solute
possible - Unsaturated Solution
- solution contains less than the maximum amount of
solute - there is no undissolved solute present
- Supersaturated Solution
- solution contains more than the maximum amount of
solute - there is no undissolved solute present
- solution is unstable
A
20
05_25.jpg
35.7 g/100 ml At 0 C
21
Factors Affecting Solubility
- Solute-Solvent Interactions
- types of solute/solvent -- polar or non-polar
- like dissolves like
- pairs of liquids which are soluble in all
proportions are miscible - pairs which are not soluble are immiscible
- The stronger the attractions between solute and
solvent the, greater the solubility
22
Conceptual Question
- Which of the following is the most soluble in
water? - a. CH3CH2OH
- b. CH3CH2CH2OH
- c. CH3CH2CH2CH2OH
- d. CH3OH
23
Factors Affecting Solubility
24
Solubilities of some Alcohols in Water
25
Factors Affecting Solubility
The number of -OH groups within a molecule
increases solubility in water.
Glucose
26
Factors Affecting Solubility
- Pressure
- solubility of gases increase with
- increasing pressure
- increasing mass
27
Factors Affecting Solubility
- Henrys Law relationship between pressure and
solubility of a gas Å - Sg kPg
- Sg is solubility of the gas
- Pg is partial pressure of gas
- k is a constant
- Carbonated beverages are bottled under gt 1
atm. As the bottle is opened,
decreases and the solubility of CO2 decreases.
Therefore, bubbles of CO2 escape from solution.
28
Calculation
- Calculate the concentration of CO2 in a soft
drink after the bottle is opened and equilibrates
at 25 C under a CO2 partial pressure of 3.0 x
10-4 atm. (Henrys Law constant 3.1 x 10-2
mol/L-atm)
29
Factors Affecting Solubility
- Temperature
- generally, solubility of solids increases with
increasing temperature
30
Factors Affecting Solubility
- solubility of gases
- decreases with
- increasing
- temperature
31
Conceptual Question
- The lowest value of the Henrys Law constant for
methane (CH4) will be obtained with _________ as
the solvent and a temperature of ___________ K? - a. C5H12, 301
- b. C6H6, 322
- c. H20, 301
- d. H2O, 301
32
Concentration
- Dilute - not a lot of solute
- Concentrated - a large amount of solute.
- strong solution - as in a strong cup of tea
- Concentration can be expressed quantitatively is
many ways - Molarity, Molality, percentage, mole fraction,
etc
33
Molarity
- The molarity is the number of moles of solute in
1 litre of solution - M moles of solute
- liters of solution
- 1M solution is fairly concentrated
- Millimolar 0.001M 1?10-3moles/L
- 1 millimole/L
- 1mM
34
Mole fraction
- If a solution consists of mA moles of A, mB moles
of B, mC moles of C, etc. - Mole fraction of A
- XA mA / (mA mB mC)
- XA XB XC 1
35
Mass percentage
- A solution that is 36 HCl by mass, contains 36 g
HCl in 100 g of solution. - Milk is not a true solution, but 2 milk refers
to 2 g of milk fat in 100 g of milk. - You may see figures on packing such as
- 3 w/w where w is short for weight (mass)
36
Calculation
- Calculate the mass percentage of NaCl in a
solution containing 1.50 g of NaCl in 50.0 g of
water.
37
You Try
- A Commercial bleaching solution contains 3.62
mass sodium hypochlorite, NaOCl. What is the
mass of NaOCl in a bottle containing 2500 g of
bleaching solution?
38
Parts per million
- Percentage refers to parts in 100. Thus 3 means
3 parts solute in 100 parts of solution. - Parts per million 1 ppm contains 1 gram of
solute per 1 million grams solution.
39
Parts per million
- A solution has a Ag ion concentration of 3 ppm
- 3 g Ag in 1,000,000 g of solution
- 3 mg Ag in 1,000 g of solution
- 1,000 g of water solution has a volume of
approximately 1 litre - So, our solution has 3 mg Ag / litre
- ppm is approximately the same as mg/L
40
Calculation
- A 2.5 g sample of groundwater was found to
contain 5.4 ?g of Zn2. What is the concentration
of Zn2 in ppm?
41
Molality
- Molality (m) moles of solute per kilogram of
solvent
42
Molality
43
Molarity
44
Calculation
- A solution is made containing 7.5 g CH3OH in 245
g H2O. - Calculate the mole fraction
- Calculate the molality
- Calculate the mass
45
Calculation
- Ascorbic Acid, (vitamin C, C6H8O6) is a
water-soluble vitamin. A solution containing 80.5
g of ascorbic acid dissolved in 210 g of water
has a density of 1.22 g/ml at 55 C. Calculate a)
mass percentage - b) mole fraction
- c) molality
- d) molarity
46
Calculation
- The density of toluene (C7H8) is 0.867 g/ml, and
the density of thiophene (C4H4S) is 1.065 g/ml. A
solution is made by dissolving 10.0 g of
thiophene in 250.0 ml of toluene. - a) Calculate the mole fraction of thiophene
- b) calculate the molality of thiophene
47
Calculation
- Caffeine (C8H10N4O2) is a stimulant found in
coffee and tea. If a solution of caffeine in
chloroform (CHCl3) as a solvent has a
concentration of 0.0750 m calculate - a) caffeine by mass
- b) mole fraction of caffeine
48
Colligative Properties
- Properties that depend on solute concentration
(quantity) not the kind of solute - Lowering the vapor pressure
- Freezing Point Depression
- Boiling-Point Elevation
- Osmosis
49
09_10.jpg
Lowering the Vapor Pressure
Why do you think the liquid levels in the two
beakers change over time?
Seawater
Distilled Water
Seawater
50
Lowering the Vapor Pressure
- Addition of a nonvolatile solute lowers the vapor
pressure of the solvent - Extent of lowering depends on concentration of
solute, described by Raoults Law - Raoults Law The equilibrium vapor pressure of
the solvent over the solution is directly
proportional to the mole fraction of the solvent
in the solution - Psolvent XsolventPsolvent
51
Lowering the Vapor Pressure
- Glycerin (C3H8O3) is a nonvolatile
non-electrolyte with a density of 1.26 g/ml at 25
C. Calculate the vapor pressure at 25 C of a
solution made by adding 50.0 ml of glycerin to
500.0 ml of water. (The vapor pressure of pure
water at 25 C is 23.8 torr.)
52
Conceptual Question
- A 0.100 m solution of which one of the following
solutes in water will have the lowest vapor
pressure? - KClO4
- Ca(ClO4)2
- Al(ClO4)3
- Sucrose
- NaCl
53
What does this say about the boiling point of the
soltuion?
At the Normal Boiling Point of a pure liquid,
vapor pressure of solution is lt 1 atm.
54
Boiling-Point Elevation
The boiling point of a solution is higher than
that of a pure liquid
Animation
55
Freezing Point Depression
- The freezing point of a solution is lower than
that of a pure solvent
56
Boiling-Point Elevation and Freezing Point
Depression
- Boiling Point Elevation
- dircetly proportional to the molality of the
solute - DTb Kb msolute
- Kb is a constant, m is molality
- Freezing Point Depression
- DTf Kf msolute
- Kf is a constant, m is molality
57
Calculations
- The concentration of table sugar in a sucrose
solution is 0.50 m. Calculate the freezing-point
for this solution. (Kf of water is 1.86 C/m)
58
More Calculations
- An environmentally concerned graduate student
adds the more earth-friendly coolant, propylene
glycol (C3H3O2, density 1.038) to her cars
radiator, resulting in a final composition of her
radiator fluid equaling 30 (v/v) ethylene glycol
(C2H6O2, d1.114 g/ml) and 20 (v/v) propylene
glycol in water. At what temperature will her
engine boil over? (Kb 0.50 C/m)
59
Conceptual Question
- Which of the following will have the lowest
freezing point? - Pure H2O
- Aqueous glucose (0.60 m)
- Aqueous sucrose (0.60 m)
- Aqueous FeI3 (0.24 m)
- Aqueous KF (0.50 m)
60
Osmosis
- Why do red blood cells sometimes either shrivel
or rupture?
61
Osmosis
- The net movement of solvent through a
semi-permeable membrane from a dilute to a more
concentrated solution.
62
Osmosis
- Eventually the pressure difference between the
arms stops osmosis.
Animation
63
Osmosis
- Osmotic pressure(?) - The pressure required to
stop osmosis - M - molarity of the solution
- R - gas constant (0.08206 L(atm)/mol(K))
- T - temperature in Kelvin
64
Osmosis through the semi-permeable membrane of a
red blood cell
having the same osmotic pressure
65
Osmosis through the semi-permeable membrane of a
red blood cell
having a higher osmotic pressure
66
Osmosis through the semi-permeable membrane of a
red blood cell
having a lower osmotic pressure
67
Osmosis
- The average osmotic pressure of blood is 7.7 atm
at 25 C. What concentration of glucose (C6H12O6)
will be isotonic with blood?
68
Fractional Distillation of Benzene and Toluene
69
Fractional Distillation of Benzene and Toluene
70
Fractional Distillation of Benzene and Toluene
71
(No Transcript)