Interactions between molecules - PowerPoint PPT Presentation
1 / 7
Title:
Interactions between molecules
Description:
Comparison of intermolecular forces with intramolecular forces responsible for chemical bonding ... of the attractions is responsible for the minimum of the ... – PowerPoint PPT presentation
Number of Views:28
Avg rating:3.0/5.0
Title: Interactions between molecules
1
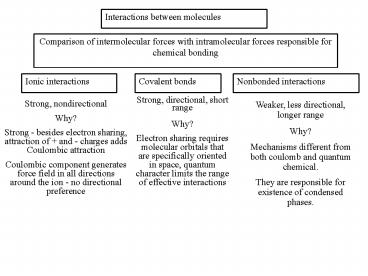
Interactions between molecules
Comparison of intermolecular forces with
intramolecular forces responsible for chemical
bonding
Ionic interactions
Covalent bonds
Nonbonded interactions
Strong, directional, short range Why? Electron
sharing requires molecular orbitals that are
specifically oriented in space, quantum character
limits the range of effective interactions
Strong, nondirectional Why? Strong - besides
electron sharing, attraction of and - charges
adds Coulombic attraction Coulombic component
generates force field in all directions around
the ion - no directional preference
Weaker, less directional, longer
range Why? Mechanisms different from both coulomb
and quantum chemical. They are responsible for
existence of condensed phases.
2
Quantitative description of bonded and nonbonded
attractions is possible using the potential
energy. It is the part of the total energy of the
system that is dependent only on the position
(distance) of interacting species (and not on
their kinetic energy). Existence of the
attractions is responsible for the minimum of the
dependence potential energy on the distance
between the interacting atoms and molecules
3
DIPOLE-DIPOLE ATTRACTION
This is how the configuration of two HCl
molecules that has minimal energy is developing
in time from the original unfavorable
orientation. The scheme was generated using
theoretical calculational method that takes into
account properties of atoms in molecule and
minimizes the total energy of the system of these
two molecules. The iso-contour lines in the
minimized structure represent the charge density
in the final minimum energy system (HCl)2
4
ION - DIPOLE ATTRACTION
Important in systems in which ions (cations or
anions) are surrounded by (solvent) molecules
with permanent electrical dipole. Then the
electrostatic field of the ion orients the
solvent molecule favorably
Anion (green) orientation of water molecules gtgt
in H2O O is more electronegative - on H there is
lack of electrons - partially positive charge,
favors to be close to - charge of the anion
Cation (blue) orientation of water molecules gtgt
in H2O O is more electronegative - there is
excess of electrons on O - partially negative
charge on O, favors to be close to - charge of
the cation
5
Ion - Induced dipole attraction
If an ion approaches the (electroneutral) atom,
the force field of the ion charge modifies the
electron distribution surrounding even
electro-neutral atom (cation attracts electrons,
anion repels them. This redistribution of the
electrons generates positive and negative partial
charges in the electroneutral molecule that in
turn represent induced dipole. This induced
dipole then interacts favorably with the charge
of the ion.
-
-
-
6
Van der Waals forces
7
Hydrogen bonding
Hydrogen bonding involves H covalently bonded to
N,O, F on one molecule (water, H on O) and free
electron pair on the second molecule (N-methyl
amide). H is shared by the two molecules and by
this the weaker analog of covalent bond is
formed. Note the nonzero electron charge density
between the water O and amide O in the last
computationally optimized structure