Aldehydes - PowerPoint PPT Presentation
1 / 57
Title:
Aldehydes
Description:
Apheloria corrugata, a millipede, discharges poisonous HCN at attackers. The millipede secretes the mandelonitrile molecule and another enzyme that ... – PowerPoint PPT presentation
Number of Views:130
Avg rating:3.0/5.0
Title: Aldehydes
1
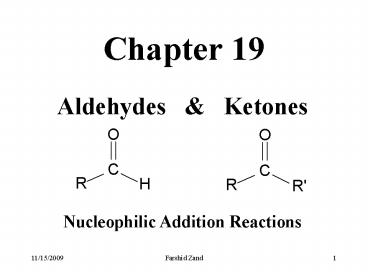
Chapter 19
Aldehydes Ketones Nucleophilic Addition
Reactions
2
Aldehyde Nomenclature
- Find the longest chain that contains the
aldehyde. - The suffix al replaces the e of the
corresponding alkane. - The carbonyl carbon receives the lowest number.
- Branches are named using the IUPAC system
- Complex aldehydes (-CHO is attached to a ring)
use the suffix -carbaldehyde
3
Examples
2-Ethyl-4-methylpentanal
4
Common Names of Aldehydes
5
Ketone Nomenclature
- Find the longest chain that contains the ketone
(it MUST NOT BE at the end) - Use the base hydrocarbon name drop the e and
replace with one. - The carbonyl group has to be assigned the lowest
possible number. - In complex molecules, the carbonyl group can be
named as a prefix with the term oxo-
6
Refering as Subsituents
- RCO-
Acyl - CH3CO-
Acetyl - -CHO
Formyl - C6H5CO-
Benzoyl - In complex molecules, the carbonyl group can be
named as a prefix with the term oxo- - methyl 3-oxohexanoate
7
Ketone Nomenclature Examples
3-Hexanone
4-Hexen-2-one
2,4 Hexanedione
8
Common Names of Some Ketones
Acetone
Acetophenone
Benzophenone
9
Synthesis of Aldehydes
- Oxidation of 1 alcohols
10
Synthesis of Aldehydes
- Oxidative cleavage of alkenes w/ O3, Zn, CH3COOH
11
Synthesis of Aldehydes
- Partial reduction of certain carboxylic acid
derivatives
12
Synthesis of Ketones
- Oxidation of 2 alcohols w/ PCC and base
13
Synthesis of Ketones
- Ozonolysis of alkenes, if one of the unsaturated
carbon atoms is disubstituted.
14
Synthesis of Ketones
- Friedel-Crafts acylation aryl
ketones
15
Synthesis of Ketones
- Hydration of terminal alkynes methyl
ketones
16
Synthesis of Ketones continue
17
Synthesis of Ketones
- From certain carboxylic acid derivatives using a
Gilman reagent (R2Cu-Li)
18
Oxidation of Aldehydes and Ketones
- aldehydes- readily oxidized to form carboxylic
acids - Ketones-inert but can be with hot alkaline KMnO4
- REASON aldehydes have a CHO proton that can be
removed during oxidation ketones dont. - Oxidizing agents KMnO4
- HNO3
- CrO3 in acidic conditions
- Tollens reagent (Ag2O) in aqueous
ammonia
19
Aldehyde Oxidation
- Occur through intermediate 1,1-diols, or hydrates.
H2O
An aldehyde
A hydrate
A carboxylic acid
20
Mechanism of Aldehyde Oxidation
21
Mechanism of Aldehyde Oxidation continue
22
Ketone Oxidation
- Inert to most oxidizing agents
- Ketones undergo slow cleavage when treated with
hot alkaline KMnO4
1.
2.
23
Nucleophilic Addition Rxns of Aldehydes and
Ketones
- Nucleophile attacks the electrophilic CO carbon
from a direction 45 to the plane of the
carbonyl group. - At the same time Rehybridization of the
carbonyl carbon from sp2 to sp3 occurs, an
electron pair from the carbon-oxygen double bond
moves toward the electronegative oxygen atom, and
a tetrahedral alkoxide ion intermediate is
produced.
24
Attacking Nucleophiles
- Can be negatively charged or neutral at the
reaction site
- Negatively charged Nucleophiles
- HO- (hydroxide ion)
- H- (hydride ion)
- R3C- (a carbanion)
- RO- (an alkoxide ion)
- CN- (cyanide ion)
- Neutral Nucleophiles
- H2O (water)
- ROH (an alcohol)
- H3N (ammonia)
- RNH2 (an amine)
25
Nucleophilic Addition Rxns of Aldehydes and
Ketones
- Formation of an alcohol
26
Nucleophilic Addition Rxns of Aldehydes and
Ketones
- Elimination of the carbonyl oxygen atom at HO-
or H2O to give a product with CNu double bond.
27
Relative Reactivity of Aldehydes and Ketones
- Reactivity in nucleophilic addition rxns
- Aldehydes gtgtgt ketones
- aliphatic aldehydes gtgtgt aromatic aldehydes
28
Steric Reason why Aldehydes are More Reactive
than Ketones
- nucleophile is able to approach aldehydes more
readily because it only has 1 large substituent
bonded to the CO carbon, vs. 2 in ketones. - transition state for the aldehyde rxn is
therefore less crowded and has lower energy. - aldehyde
ketone
29
Electronic Reason why Aldehydes are More Reactive
than Ketones
- greater polarization of aldehyde carbonyl group
- aldehyde is more electrophilic and more reactive
than ketones.
2carbocation (more stable, less
reactive)
Ketone (more stabilization of ?, less
reactive)
1 carbocation (less stable,
more reactive)
Aldehyde (less stabilization of ?, more
reactive)
?-
?-
?
?
30
Why aromatic aldehydes gtgtgt aliphatic aldehydes
- The electon-donating resonance effect of the
aromatic ring makes the carbonyl group less
electrophilic than the carbonyl group of the
aliphatic aldehyde.
31
Why aromatic aldehydes gtgtgt aliphatic
aldehydesexample
- Comparing electrostatic potential maps of
formaldehyde and benzaldehyde, shows that the
carbonyl carbon atom is less positive in the
aromatic aldehyde - formaldehyde benzaldehyde
32
Nucleophilic Addition of H2O Hydration
- Aldehydes and ketones react with water to yield a
geminal diol. This hydration process is
reversible. - Nucleophilic addition of water is catalyzed by
acid and base. - Base-catalyzed
- Acid-catalyzed
33
Nucleophilic Addition of HCN Cyanohydrin
Formation
- Aldehydes and unhindered ketones react with HCN
to yield cyanohydrins. This formation is
reversible and base-catalyzed.
- Cyanohydrins formation is unusual due to the
addition of protic acid to a carbonyl group,
but useful because of further chemistry. - Reduced with LiAlH4, yielding primary amine.
- Hydrolyzed with hot aqueous acid, yielding
carboxylic acid.
34
Nucleophilic Addition of Grignard Hydride
Reagents Alcohol Formation
- Grignard reagents R-MgX, strongly polarized
reacts with an acid-base behavior.
Nucleophilic addition of a carbanion to an
aldehyde or ketone, followed by protonation of
alkoxide intermediate, yields an alcohol.
- Addition of hydride ion, from LiAlH4 or NaBH4,
and water or aqueous acid yields an alcohol.
35
Nucleophilic Addition of AminesImine and
Enamine Formation
- Difference between imine and enamine is the CN
bond and CC bond.
36
Nucleophilic Addition of AminesMechanism of 1º
amines forming Imine
37
Nucleophilic Addition of AminesMechanism of 2º
amines forming Enamine
38
Nucleophilic Addition of HydrazineWolff-Kishner
Reaction
- Addition of hydrazine converts aldehyde/ketone to
an alkane. An intermediate hydrazone forms,
followed by base catalyzed double bond migration,
loss of N2 gas, finally protonation yields an
alkane.
39
Nucleophilic Addition of Alcohols Acetal
Formation
- Acetals and Ketals are formed by reacting two
equivalents of an alcohol with an aldehyde or
ketone, in the presence of an acid catalyst. - Hemiacetals and Hemiketals are formed by reacting
only one equivalent of alcohol with the aldehyde
or ketone in the presence of an acid catalyst.
Further reaction with a second alcohol forms the
acetal or ketal. - A diol, with two OH groups on the same molecule,
can be used to form cyclic acetals. - All steps in acetal/ketal formation are
reversible.
40
- Acetal
Ketal - Hemiacetal
Hemiketal
41
Mechanism of Acetal Formation
42
Nucleophilic Addition of Phosphorus Ylides The
Wittig Reaction
- Converts an aldehyde/ketone into an alkene.
- A phosphorus ylide(aka phosphorane),
,
- acts as the nucleophile to attack the
carbonyl carbon - and yields a four-membered ring, dipolar
intermediate - called the betaine.
- The betaine decomposes spontaneously to yield an
- alkene and a triphenylphosphine oxide.
- Can produce monosubstituted, disubstituted, and
- trisubstituted alkenes.
43
Mechanism of the Witting Reaction
44
The Canizzaro Reaction
- Requires two equivalents of an aldehyde and a
heated aqueous base. - Produces a 11 mixture of carboxylic acid and
alcohol. - Limited to aldehydes such as formaldehyde and
benzaldehyde, which have no hydrogens on carbon
next to carbonyl. - Results in simultaneous oxidation and reduction,
disproportionation. - Steps
- 1. Nucleophillic addition of OH- to first
aldehyde forms a tetrahedral - intermediate.
- 2. Tetrahedral intermediate then expels the
hydride ion as a leaving - group.
- 3. The second aldehyde picks up the hydride
ion. - 4. Oxidation of second product yields the
acid while reduction of - the first product yields an alcohol.
45
Mechanism of Cannizzaro Reaction
46
Conjugate Nucleophilic Addition to
alpha,beta-Unsaturated Aldehydes and Ketones
- Direct addition (aka 1,2 addition) occurs when a
nucleophile attacks the carbon in the carbonyl
directly. - Conjugate addition (aka 1,4 addition) occurs when
the nucleophile attacks the carbonyl indirectly
by attacking the second carbon away from the
carbonyl group, called the beta carbon, in an
unsaturated aldehyde or ketone. - Conjugate addition reactions form an initial
product called an enolate, which is protonated on
the carbon next to the carbonyl, the alpha
carbon, to give the final saturated
aldehyde/ketone product. - Conjugate addition can be carried out with
nucleophiles such as primary amines, secondary
amines, and even alkyl groups like in
organocopper reactions. - It is the carbonyl that activates the conjugated
CC double bond for addition which would
otherwise not react.
47
Conjugate (1,4) addition mechanism
48
Some Biological Nucleophilic Addition Reactions
- Living organisms use nucleophilic addition
reactions involving aldehydes and ketones in
nature.
- Examples
- -In Metabolism Breakdown of alanine
- -In Defense Secretion of poison by the
millipede
49
A Nucleophilic Addition Reaction Metabolism
- The human body uses the amino acid alanine to
react with pyridoxal phosphate, an aldehyde, in a
metabolic reaction to produce an imine.
50
A Nucleophilic Addition Reaction Defense
- Apheloria corrugata, a millipede, discharges
poisonous HCN at attackers. - The millipede secretes the mandelonitrile
molecule and another enzyme that breaks it down
into benzaldeyde and HCN.
51
Spectroscopy of Aldehydes and Ketones
- Aldehydes and Ketones show characteristic
absorptions in Infrared Spectroscopy, Nuclear
Magnetic Resonance Spectroscopy, and Mass
Spectrometry.
52
Infrared Spectroscopy of Aldehydes/Ketones
- Aldehydes/Ketones show a strong CO bond
absorption at 1660-1770 cm-1. - Aldehydes show two characteristic absorptions in
the - 2720-2820 cm-1 range.
- Conjugation of an aldehyde lowers absorption
position. - Angle strain in the carbonyl group of a ketone
caused by reducing the ring size of cyclic
ketones raises absorption position.
53
IR of Benzaldehyde and Cyclohexanone
54
Nuclear Magnetic Resonance of Aldehydes/Ketones
- H NMR
- Aldehyde protons absorb near 10 ppm.
- Hydrogens next to the carbonyls of aldehydes and
ketones are slightly deshielded and absorb near 2
to 2.3 ppm. - Methyl ketones show a sharp three-proton singlet
near 2.1 ppm.
- Aldehyde and ketone carbonyl carbons show
absorptions between 190 to 215 ppm. - Saturated aldehyde or ketone carbons absorb in
the 200 to 215 ppm range. - Aromatic and a,b-unsaturated carbonyl carbons
absorb in the 190 to 200 ppm range.
- C NMR
55
H NMR of Acetaldehyde
56
Mass Spectrometry of Aldehydes/Ketones
- Alipatic aldehydes and ketones that have
hydrogens on their gamma carbon atoms undergo
McLafferty rearrangement.
57
Mass Spectrum of 5-methyl-2-hexanone