Le Chateliers Principle: - PowerPoint PPT Presentation
1 / 6
Title:
Le Chateliers Principle:
Description:
Increasing concentration of a reactant causes the equilibrium to ... High pressure and low temperature favour product. Application of Le Chatelier's Principle: ... – PowerPoint PPT presentation
Number of Views:109
Avg rating:3.0/5.0
Title: Le Chateliers Principle:
1
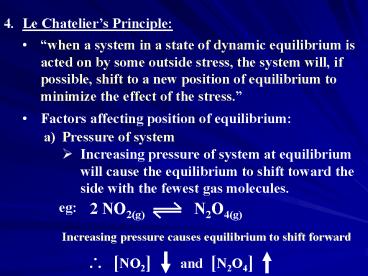
- Le Chateliers Principle
- when a system in a state of dynamic equilibrium
is acted on by some outside stress, the system
will, if possible, shift to a new position of
equilibrium to minimize the effect of the stress.
- Factors affecting position of equilibrium
- Pressure of system
- Increasing pressure of system at equilibrium will
cause the equilibrium to shift toward the side
with the fewest gas molecules.
eg
Increasing pressure causes equilibrium to shift
forward
2
- Concentration (partial pressure)
- Increasing concentration of a reactant causes the
equilibrium to shift right (towards products). - Increasing concentration of a product causes the
equilibrium to shift left (towards reactants).
eg
If some H2(g) is added to this system, the
equilibrium will shift forward
If some H2(g) is removed from this system, the
equilibrium will shift backward
3
- Temperature
- Increasing temperature favours the endothermic
reaction. - Decreasing temperature favours the exothermic
reaction.
eg
Increase temperature, reaction will shift forward
Decrease temperature, reaction will shift backward
- Catalysts
- Have no effect on the position of equilibrium.
- They can however cause the equilibrium to be
reached more quickly.
4
Practice
What happens to the concentration of each
substance if
- Increase NH3?
- Decrease N2?
- Decrease total pressure?
- Decrease temperature?
- Increase temperature?
5
Application of Le Chateliers Principle
The Haber Process (production of ammonia)
- High pressure and low temperature favour product.
- However, low temperature slows down the reaction.
? use high pressure, high temperature and remove
NH3 as it is produced maintaining the forward
reaction at a high rate.
6
Assignment
- Classwork
- Worksheet 25-28
- Homework
- P.556 Interpret Apply 1-5 (skip 1e,f)