Corrections from Wednesday - PowerPoint PPT Presentation
1 / 20
Title:
Corrections from Wednesday
Description:
Similar to alkanes, except. Root hydrocarbon name ends in ene ... What functional groups are present in acetaminophen? Alcohol. Secondary Amine. Ketone ... – PowerPoint PPT presentation
Number of Views:21
Avg rating:3.0/5.0
Title: Corrections from Wednesday
1
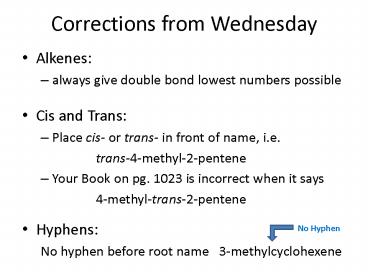
Corrections from Wednesday
- Alkenes
- always give double bond lowest numbers possible
- Cis and Trans
- Place cis- or trans- in front of name, i.e.
- trans-4-methyl-2-pentene
- Your Book on pg. 1023 is incorrect when it says
- 4-methyl-trans-2-pentene
- Hyphens
- No hyphen before root name 3-methylcyclohexene
No Hyphen
2
Alkene Nomenclature
- Similar to alkanes, except
- Root hydrocarbon name ends in ene
- Location of the double bond is indicated
- add the lowest-numbered double-bound carbon
before root name - Give double bond the lowest possible numbers
- The double bond must be included in parent chain
1-butene
2-butene
3
- Draw the structure for 4-methyl-2-pentene.
- Correction This is the correct name for the
structure. Ensure that the double bond has the
lowest possible numbers. - However, you can add cis- or trans-
- above trans-4-methyl-2-pentene
- cis-4-methyl-2-pentene ?
4
Cylic Alkene Nomenclature
- For cyclic alkenes, place double bond between C1
and C2 - Dont need to indicate position of double bond in
name - Ex Draw 3-methylcyclohexene
4-ethylcyclopentene
No Hyphen
5
Alkene Nomenclature Change
- Standard naming, before 1993
- 3-methyl-2-hexene
- In 1993, IUPAC recommended a change
- 3-methylhex-2-ene
- Your book, and this course, uses old system
- 3-methyl-2-hexene
6
- Name the following
3
4
5
6
7
1
2
Not numbered 7 ? 1 Double bond has priority
3-Heptene
6-methyl-
cis-
7
Not part of parent Double bond must be in
parent chain
- Name the following
- An approximation for this course
- Choose largest group on each side of the double
bond - If these groups are on the same side, cis-
2
3
1
4
5
6
7
2-Heptene
5-methyl-3-propyl-
cis-
8
Alkynes
- Contain a triple bond
- Named like alkenes, except suffix yne
- Example Name the following
1 2 3 4
Give the triple bond the lowest possible number
4-methyl-2-hexyne
9
Aromatic Hydrocarbons (21.3)
- Cyclic unsaturated hydrocarbons with delocalized
p electrons - Simplest example is benzene
Fig 21.11 pg 1026
10
Naming Benzene Derivatives
- Like other cyclic hydrocarbons
- use root name benzene
- When benzene is a substituent, called phenyl
11
Alternate Benzene Naming
- Ortho next to
- Meta one carbon between
- Para across
Fig 21.12 pg 1027
12
- Draw the structure for
- 1-ethyl-3-methylbenzene
- Another name m-ethylmethylbenzene
13
- Common name of aromatic compound can serve as
root name
Toluene
2-methyl-1,3,5-trinitrobenzene or 2,4,6-trinitroto
luene (TNT) (set methyl carbon as 1)
14
Hydrocarbon Derivatives (21.4)
- Hydrocarbon backbones often have atoms other than
carbon and hydrogen - These functional groups exhibit characteristic
chemistry
Cholesterol
Functional Group
15
Know how to Recognize All functional groups in
your book, table 21.4 Name Halocarbons Alcohols A
ldehydes Ketones Carboxylic Acids
16
- What functional groups are present in
acetaminophen?
Secondary Amine
Ketone
Alcohol
17
Tertiary Amine
Ester
Primary Amine
- What functional groups are present in procaine
(novocaine)?
18
Naming Halogen Substituents
Use like alkyl substituents (methyl- )
- F fluoro-
- Cl chloro-
- Br bromo-
- I iodo-
19
Naming Hydrocarbon Derivatives
- Select parent chain
- Longest chain that contains the functional group
- Name parent chain with suffix to reflect
functional group (replace e ending with suffix) - Number parent chain
- Place functional group closest to carbon 1
- Only number carbon atoms!
- Specify location of functional group if necessary
- Add substituents (like before)
20
Parent Chains
- (root name) (letters for single, multiple bond)
(suffix) - an en yn
- Hydrocarbons e (i.e. hexane hexene )
- Alcohols ol (i.e. hexanol hexenol )
- Aldehydes al
- Ketones one
- Carboxylic Acids oic acid