Bioenergetics (Overview) - PowerPoint PPT Presentation
1 / 38
Title:
Bioenergetics (Overview)
Description:
They are involved in the building up of simpler molecules into more-complex ones ... Cofactor. Polypeptide. Enzyme Saturation. Substrate. Product ... – PowerPoint PPT presentation
Number of Views:97
Avg rating:3.0/5.0
Title: Bioenergetics (Overview)
1
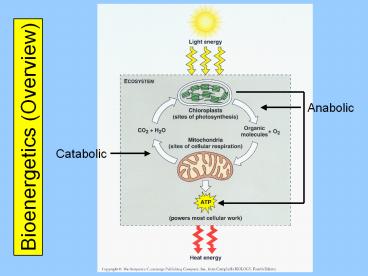
Bioenergetics (Overview)
2
Metabolism (Overview)
Metabolism Catabolism Anabolism
Catabolic reactions are energy yielding
They are involved in the breakdown of
more-complex molecules into simpler ones
Anabolic reactions are energy requiring
They are involved in the building up of simpler
molecules into more-complex ones
3
Energy Coupling in Metabolism
Catabolic Reactions provide the energy that
drives Anabolic Reactions forward
4
Organisms are Energy Transducers
First Law of Thermodynamics
Energy can be neither created nor destroyed
Therefore, energy generated in any system is
energy that has been transformed from one state
to another (e.g., chemically stored energy
transformed to heat)
Second Law of Thermodynamics
Efficiencies of energy transformation never equal
100
Therefore, all processes lose energy, typically
as heat, and are not reversible unless the system
is open the lost energy is resupplied from the
environment
Conversion to heat is the ultimate fate of
chemical energy
5
Organisms are Energy Transducers
6
Organisms are Energy Transducers
Organisms take in energy transduce it to new
forms (1st law)
As energy transducers organisms are lt100
efficient (2nd law)
Organisms employ this energy to
- Grow
- Protect Themselves
- Repair Themselves
- Compete with other Organisms
- Make new Organisms (I.e., babies)
In the process, organisms generate waste
chemicals heat
Organisms create local regions of order at the
expense of the total energy found in the
Universe!!! We are Energy Parasites!
7
Energy (a reminder)
Potential Energy
Kinetic Energy
Kinetic
8
Free Energy Spontaneity
9
Free Energy Spontaneity
Rather than lighting bulbs, in most biological
systems incoming energy is either stored or is
used to produce ATP
10
Energy Coupling via ATP (1/2)
11
Hydrolysis of ATP
12
Energy Coupling via ATP (2/2)
13
Energy Coupling by Pi Transfer
14
Exergonic Reaction (Spontaneous)
- Decrease in Gibbs free energy (-?G)
- Increase in stability
Overview
- Spontaneous (gives off net energy upon going
forward)
- Downhill (toward center of gravity well, e.g.,
of Earth)
- Movement towards equilibrium
- Coupled to ATP production (ADP phosphorylation)
- Catabolism
Endergonic Rxn (Non-Spontaneous)
- Increase in Gibbs free energy (?G)
- Decrease in stability
Overview
- Not Spontaneous (requires net input of energy to
go forward)
- Uphill (away from center of gravity well, e.g.,
of Earth)
- Movement away from equilibrium
- Coupled to ATP utilization (ATP
dephosphorylation)
- Anabolism
15
Low- (i.e., body-) Temperature Stability
To be unstable, something must have the potential
to change into something else, typically
something that possesses less free energy
To be unstable, releasing somethings ability to
change into something else must also be
relatively easy (i.e., little input energy)
Why don't energy-rich molecules, e.g.,
glucose, spontaneously degrade into CO2 and Water?
Therefore, Stability already low free energy
Alternatively, Stability high activation energy
Things, therefore, can be high in free energy but
still quite stable, e.g., glucose
16
Transition State
17
Chemical Reaction
Without Catalysts, Transition States are
Achieved via an input of Heat, i.e., Higher
Temperatures
Activation Energy ?
Activation Energy ?
a.k.a., Substrate if enzyme catalyzed
18
Chemical Reaction
Note no change in degree of spontaneity, i.e., in
?G
19
Catalyzed Reaction
At a given temperature catalyzed Rxns can run
faster because less energy is required to achieve
the transition state
20
Catalyzed Reaction
21
Induced Fit (Active Site)
The Catalysis associated with Enzymes occurs
within small regions on (or within) proteins
called Active Sites
Induced Fit not only allows the enzyme to bind
the substrate(s), but also provides a subtle
application of energy (e.g., bending chemical
bonds) that causes the substrate(s) to
destabilize into the transition state
22
Subtle Application of Energy
23
Enzyme Catalytic Cycle
- Input of Activation
- Energy
24
Mechanisms of Catalysis
(1) Active sites can hold two or more substrates
in proper orientations so that new bonds between
substrates can form
(2) Active sites can stress the substrate into
the transition state
(3) Active sites can maintain conducive physical
environments (e.g., pH)
(4) Active sites can participate directly in the
reaction (e.g., forming transient covalent bonds
with substrates)
(5) Active sites can carry out a sequence of
manipulations in a defined temporal order (e.g.,
step A ? step B ? step C)
25
Mechanisms of Catalysis
Metal Ion or Organic Molecule
Organic Cofactor
26
Enzyme Saturation
Enzyme Activity at Saturation is a function of
Enzyme Turnover Rate
27
Enzyme Saturation
28
Modification of Enzyme Activity
Even at Saturation the rate of Enzymatic
Reactions can be modified
29
More-Subtle Inhibition of Active Sites (1/2)
30
More-Subtle Inhibition (2/2)
31
Multi-Subunit Enzymes (1/2)
?
Recall that a Multi-Subunit Enzyme is a catalytic
Protein that consists of more than one Polypeptide
This is a description of Allosteric Regulation
(Inhibition)
32
Multisubunit Enzymes (2/2)
This also is a form of Allosteric Regulation
(activation)
33
Feedback Inhibition
34
Energy-Metabolism Regulation
35
Enzyme Localization
Organization of Electron Transport Chain of
Cellular Respiration Substrate ? Enzyme ?
Product ? Enzyme chains are co-localized
36
First Exam Next Wednesday
The first exam is scheduled for next Wednesday
This exam will cover chapters 2 through 6 (unit 1)
Expect same questions per class met
Thats 7 classes x (3 to 5 questions/class) 25
to 35 questions
Study over the weekend (perhaps already having
started?)
Tuesday will be a recitationbring questions!!!!
The exam will start as soon as we can get in the
room
Itll be limited in length by a need to get
people to next classes
37
First Exam Next Wednesday
Lets try to avoid the scholastic equivalent of
this!
38
Link to Next Presentation