First Law of Thermodynamics - PowerPoint PPT Presentation
1 / 6
Title:
First Law of Thermodynamics
Description:
The First Law of Thermodynamics states that energy can neither be created or ... The contents of a closed flask are heated from 25 C to 80 ... – PowerPoint PPT presentation
Number of Views:97
Avg rating:3.0/5.0
Title: First Law of Thermodynamics
1
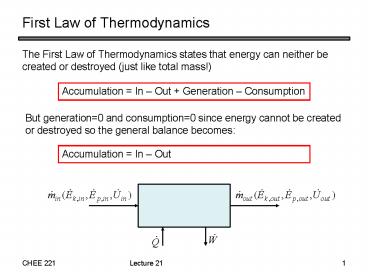
First Law of Thermodynamics
- The First Law of Thermodynamics states that
energy can neither be created or destroyed (just
like total mass!)
Accumulation In Out Generation
Consumption
But generation0 and consumption0 since energy
cannot be created or destroyed so the general
balance becomes
Accumulation In Out
2
Energy Balances on Closed Systems
- Closed System no material crosses the system
boundary over a period of time (e.g., batch
process). - General balance equation is
- Although no mass crosses the boundaries, input?0
and output?0 since energy can be transferred
across the boundary. Therefore, the balance
becomes
Accumulation Input Output
1st Law of Thermodynamics for a Closed System
(? final initial)
3
Notes on Energy Balances for a Closed System
- Possible Simplifications
- if Tsystem Tsurroundings, then Q 0 since no
heat is being transferred due to temperature
difference - if the system is perfectly insulated, then Q 0
(system is adiabatic) since no heat is being
transferred between the system and the
surroundings - if system is not accelerating, then ?Ek 0
- if system is not rising or falling, then ?Ep 0
- if energy is not transferred across the system
boundary by a moving part (e.g., piston,
impeller, rotor), then W 0 - if system is at constant temperature (system is
isothermal), no phase changes or chemical
reactions are taking place, and only minimal
pressure changes, then ?U 0
4
Examples of Closed Systems
Example 1 Heating water in a sealed container
Example 2 Compressing a gas in a cylinder.
5
Problem (Problem 7.9 FR)
- Write and simplify the closed-system energy
balance for each of the following processes, and
state whether nonzero heat and work terms are
positive or negative. Begin by defining the
system. - The contents of a closed flask are heated from
25?C to 80?C. - A tray filled with water at 20?C is put into a
freezer. The water turns into ice at -5?C.
(Note When a substance expands it does work on
its surroundings and when it contracts the
surroundings do work on it.) - A chemical reaction takes place in a closed
adiabatic (perfectly insulated) rigid container.
- Repeat part (c), only suppose the reactor is
isothermal rather than adiabatic and that when
the reaction was carried out adiabatically the
temperature in the reactor increased.
6
Problem (Example 7.3-1)
- A gas is contained in a cylinder fitted with a
movable piston. The initial gas temperature is
25?C. - The cylinder is placed in boiling water with the
piston held in a fixed position by a clamp. Heat
in the amount of 2.00 kcal is transferred to the
gas, which equilibrates at 100?C (and a higher
pressure). The piston is then released, and the
gas does 100 J of work in moving the piston to
its new equilibrium position. The final gas
temperature is 100 ?C. - Write the energy balance equation for each of the
two stages of this process, and in each case,
solve for the unknown energy term in the
equation. Consider the gas in the cylinder to be
the system, neglect the change in potential
energy of the gas as the piston moves vertically,
and assume the gas behaves ideally. Express all
energies in Joules.