AcidBase Reactions: HCl example - PowerPoint PPT Presentation
1 / 15
Title:
AcidBase Reactions: HCl example
Description:
Bronsted-Lowry Acids: substances that donate a proton. Bronsted-Lowry Bases: substances that accept a proton. What about weak acids? ... – PowerPoint PPT presentation
Number of Views:66
Avg rating:3.0/5.0
Title: AcidBase Reactions: HCl example
1
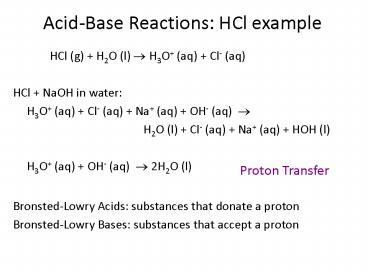
Acid-Base Reactions HCl example
- HCl (g) H2O (l) ? H3O (aq) Cl- (aq)
- HCl NaOH in water
- H3O (aq) Cl- (aq) Na (aq) OH- (aq) ?
- H2O (l) Cl- (aq) Na (aq) HOH
(l) - H3O (aq) OH- (aq) ? 2H2O (l)
Proton Transfer
Bronsted-Lowry Acids substances that donate a
proton Bronsted-Lowry Bases substances that
accept a proton
2
What about weak acids?
- CH3COOH (aq) NaOH (aq) ? CH3COONa (aq) H2O
(l) - Total ionic?
- Net Ionic?
- Proton transfer occurs Ion appears undissociated
3
Oxidation-Reduction Reactions
- Net movement of electrons from one reactant to
another - 2 Mg (s) O2 (g) --gt 2 MgO (s)
- Charge on magnesium? Oxygen?
- LEO GER
- Reducing agent? Oxidizing agent?
4
Oxidation Numbers (O.N.)
- Any element has O.N. 0
- 1A(1) ions 1 2A(2) ions 2
- H ion can only be 1 or -1
- Oxygen only -2 (unless in peroxide or with F)
- Examples ZnCl2
- sulfur trioxide
- potassium permanganate
5
Redox Reaction?
- CaO (s) CO2 (g) ? CaCO3 (s)
- Ca before 2 after 2
- O before 2- after 2-
- C before 4 after 4
6
Balancing Redox Equations
- e- lost e- gained
- Oxidation Number Method
- Assign O.N.s
- Identify reduced and oxidized species
- Compute e- lost and gained
- Multiply so e- lost e- gained
- Complete balancing
Example PbS (s) O2 (g)
7
Balancing Redox Equations
- Half-Reaction Method
- Assign O.N.s
- Write out each half reaction
- Multiply until e- lost e- gained
- Complete balancing
8
Elements in Redox Reactions
- When an atom or molecule that starts as an
element becomes part of a compound redox
reaction - Combination Reactions
- Decomposition Reactions
- Displacement Reactions
9
Combination Reactions
- Metal nonmetal
- 4 Al (s) 3 O2 (g) ? 2 Al2O3 (s)
- Nonmetal nonmetal
- N2 (g) 3H2 (g) ? 2 NH3 (g)
- Compounds elements
- 2 NO (g) O2 (g) ? 2 NO2 (g)
10
Decomposition Reactions
- Thermal Energy (Heat)
- 2 KClO3 (s) ? 2 KCl (s) 3 O2 (g)
- Electrical Energy (Electrolysis)
- 2 H2O (l) 2H2 (g) O2 (g)
11
Displacement Reactions
- Double displacement
- Precipiatation
- Acid-base
- Single displacement
- Redox
- How do you know what will displace what?
- Activity Series (metals halogens)
12
Activity Series of Metals
- Metal water ? metal hydroxide H2
- Metal metal halide
X-
X-
M
M
M
M
?
Strong reducing agent - more reactive
13
Activity Series of Halogens
- F2 gt Cl2 gt Br2 gt I2
I2
I-
Cl2
Cl-
M
M
2
2
?
2
Strong oxidizing agent - more reactive
14
Combustion Reactions
- Always redox O2 becomes O 2-
- O2 always a reactant
- H2O and CO2 common products
15
Reversibility
- When it appears that no more product being made
equilibrium - Dynamic (not static)
- Example decomposition of CaCO3 (s)
- Weak acids/bases often at equilibrium