Polyprotic AcidBase Equilibria - PowerPoint PPT Presentation
1 / 38
Title:
Polyprotic AcidBase Equilibria
Description:
Amino acids are zwitterion molecule with both positive and negative charge ... Neutral zwitterion. Only ions are H2A , A-, H and OH ... – PowerPoint PPT presentation
Number of Views:927
Avg rating:3.0/5.0
Title: Polyprotic AcidBase Equilibria
1
Polyprotic Acid-Base Equilibria
- Introduction
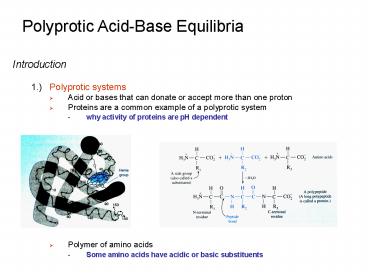
- 1.) Polyprotic systems
- Acid or bases that can donate or accept more than
one proton - Proteins are a common example of a polyprotic
system - why activity of proteins are pH dependent
- Polymer of amino acids
2
Polyprotic Acid-Base Equilibria
- Introduction
- 1.) Polyprotic systems
- Amino acids
- Carboxyl group is stronger acid of ammonium group
- R is different group for each amino acid
- Amino acids are zwitterion molecule with both
positive and negative charge
(basic)
(acidic)
Overall charge is still neutral
3
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 2.) Multiple Equilibriums
- Illustration with amino acid leucine (HL)
- Equilibrium reactions
high pH
low pH
Carboxyl group Loses H
ammonium group Loses H
Diprotic acid
4
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 2.) Multiple Equilibriums
- Equilibrium reactions
Diprotic base
Relationship between Ka and Kb
5
pKa of carboxy and ammonium group vary depending
on substituents
Largest variations
6
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Three components to the process
- Acid Form H2L
- Basic Form L-
- Intermediate Form HL
low pH
high pH
Carboxyl group Loses H
ammonium group Loses H
7
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Acid Form (H2L)
- Illustration with amino acid leucine
- H2L is a weak acid and HL is a very weak acid
K21.80x10-10
K14.70x10-3
Assume H2L behaves as a monoprotic acid
8
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- 0.050 M leucine hydrochloride
K14.70x10-3
H
Determine H from Ka
Determine pH from H
Determine H2L
9
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Acid Form (H2L)
What is the concentration of L- in the
solution? L- is very small, but non-zero.
Calculate from Ka2
Approximation H HL, reduces Ka2 equation
to L-Ka2
Validates assumption
10
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- For most diprotic acids, K1 gtgt K2
- Assumption that diprotic acid behaves as
monoprotic is valid - Ka Ka1
- Even if K1 is just 10x larger than K2
- Error in pH is only 4 or 0.01 pH units
- Basic Form (L-)
- L- is a weak base and HL is an extremely weak base
Assume L- behaves as a monoprotic base
11
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- 0.050 M leucine salt (sodium leucinate)
Determine OH- from Kb
Determine pH and H from Kw
Determine L-
12
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Basic Form (L-)
What is the concentration of H2L in the
solution? H2L is very small, but non-zero.
Calculate from Kb2
Validates assumption OH- HL,
Fully basic form of a diprotic acid can be
treated as a monobasic, KbKb1
13
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Intermediate Form (HL)
- More complicated HL is both an acid and base
- Amphiprotic can both donate and accept a proton
- Since Ka gt Kb, expect solution to be acidic
- Can not ignore base equilibrium
- Need to use Systematic Treatment of Equilibrium
14
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Intermediate Form (HL)
Step 1 Pertinent reactions
Step 2 Charge Balance
Step 3 Mass Balance
Step 4 Equilibrium constant expression (one
for each reaction)
15
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Intermediate Form (HL)
Step 6 Solve
Substitute Acid Equilibrium Equations into charge
balance
All Terms are related to H
Multiply by H
16
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Intermediate Form (HL)
Step 6 Solve
Factor out H2
Rearrange
17
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Intermediate Form (HL)
Step 6 Solve
Multiply by K1 and take square-root
Assume HLF, minimal dissociation (K1 K2 are
small)
18
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Intermediate Form (HL)
Step 6 Solve
Calculate a pH
19
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Intermediate Form (HL)
Step 7 Validate Assumptions
Assume HLF0.0500M, minimal dissociation (K1
K2 are small).
Calculate L- H2L from K1 K2
Assumption Valid
HL0.0500M gtgt 9.36x10-6 H2L 1.02x10-5 L-
20
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Intermediate Form (HL)
- Summary of results
- L- H2L ? two equilibriums proceed equally
even though KagtKb - Nearly all leucine remained as HL
- Range of pHs and concentrations for three
different forms
21
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Simplified Calculation for the Intermediate Form
(HL)
Assume K2F gtgt Kw
Assume K1ltlt F
22
Polyprotic Acid-Base Equilibria
- Diprotic Acids and Bases
- 3.) General Process to Determine pH
- Simplified Calculation for the Intermediate Form
(HL)
Cancel F
Take the -log
pH of intermediate form of a diprotic acid is
close to midway between pK1 and pK2
Independent of concentration
23
Polyprotic Acid-Base Equilibria
- Diprotic Buffers
- 1.) Same Approach as Monoprotic Buffer
- Write two Henderson-Hasselbalch equations
- Both Equations are always true
- Solution only has one pH
- Choice of equation is based on what is known
- H2A and HA- known use pK1 equation
- HA- and A2- known use pK2 equation
24
Polyprotic Acid-Base Equilibria
- Diprotic Buffers
- 1.) Example 1
- How many grams of Na2CO3 (FM 105.99) should be
mixed with 5.00 g of NaHCO3 (FM 84.01) to produce
100 mL of buffer with pH 10.00?
FM 62.03
FM 84.01
FM 105.99
25
Polyprotic Acid-Base Equilibria
- Diprotic Buffers
- 1.) Example 2
- How many milliliters of 0.202 M NaOH should be
added to 25.0 mL of 0.0233 M of salicylic acid
(2-hydroxybenzoic acid) to adjust the pH to 3.50?
26
Polyprotic Acid-Base Equilibria
- Polyprotic Acids and Bases
- 1.) Extend Treatment of Diprotic Acids and Bases
to Polyprotic Systems - Equilibria for triprotic system
- For a polyprotic system, would simply contain n
such equilibria
Acid equilibria
Base equilibria
27
Polyprotic Acid-Base Equilibria
- Polyprotic Acids and Bases
- 1.) Extend Treatment of Diprotic Acids and Bases
to Polyprotic Systems - Rules for triprotic system
- H3A is treated as a monoprotic acid, Ka K1
- H2A- is treated similarly as an intermediate form
of a diprotic acid - HA2- is also treated similarly as an intermediate
form of a diprotic acid - Surrounded by H2A- and A3-
- Use K2 K3, instead of K1 K2
28
Polyprotic Acid-Base Equilibria
- Polyprotic Acids and Bases
- 1.) Extend Treatment of Diprotic Acids and Bases
to Polyprotic Systems - Rules for triprotic system
Treat as Monoprotic acid
Treat as Intermediate Forms
Treat as Intermediate Forms
Treat as Monoprotic base
For more complex system, just have additional
intermediate forms in-between the two monoprotic
acid and base forms at ends
End Forms of Equilibria that Bracket Reactions
are Treated as Monoprotic
Use Kas that bracket or contain form, K1 K2
Use Kas that bracket or contain form, K2 K3
29
Polyprotic Acid-Base Equilibria
- Polyprotic Acids and Bases
- 2.) Example
- How many milliters of 1.00 M KOH should be added
to 100 mL of solution containing 10.0 g of
histidine hydrochloride (His.HCl FM 191.62) to
get a pH of 9.30?.
30
Polyprotic Acid-Base Equilibria
- Polyprotic Acids and Bases
- 3.) Which is the Principal Species?
- Depends on the pH of the Sample and the pKa
values - At pKa, 11 mixture of HA and A-
- For monoprotic, A- is predominant when pH gt pKa
- For monoprotic, HA is predominant when pH lt pKa
- Similar for polyprotic, but several pKa values
Triprotic acid
Diprotic acid
Determine Principal Species by Comparing the pH
of the Solution with the pKa Values
31
Polyprotic Acid-Base Equilibria
- Polyprotic Acids and Bases
- 4.) Fractional Composition Equations
- Fraction of Each Species at a Given pH
- Useful for
- Acid-base titrations
- EDTA titrations
- Electrochemical equilibria
- Combine Mass Balance and Equilibrium Constant
Rearrange
32
Polyprotic Acid-Base Equilibria
- Polyprotic Acids and Bases
- 4.) Fractional Composition Equations
- Combine Mass Balance and Equilibrium Constant
Recall fraction of molecule in the form HA is
Divide by F
Fraction in the form HA
Fraction in the form A-
33
Polyprotic Acid-Base Equilibria
- Polyprotic Acids and Bases
- 4.) Fractional Composition Equations
- Diprotic Systems
- Follows same process as monoprotic systems
Fraction in the form H2A
Fraction in the form HA-
Fraction in the form A2-
34
Polyprotic Acid-Base Equilibria
- Isoelectric and Isoionic pH
- 1.) Isoionic point is the pH obtained when the
pure, neutral polyprotic acid HA is dissolved in
water - Neutral zwitterion
- Only ions are H2A, A-, H and OH-
- Concentrations are not equal to each other
pH obtained by simply dissolving alanine
Isoionic point
Remember Net Charge of Solution is Always Zero!
35
Polyprotic Acid-Base Equilibria
- Isoelectric and Isoionic pH
- 2.) Isoelectric point is the pH at which the
average charge of the polyprotic acid is 0 - pH at which H2A A-
- Always some A- and H2A in equilibrium with HA
- Most of molecule is in uncharged HA form
- To go from isoionic point (all HA) to isoelectric
point, add acid to decrease A- and increase
H2A until equal - pK1 lt pK2 ? isoionic point is acidic? excess A-
Remember Net Charge of Solution is Always Zero!
36
Polyprotic Acid-Base Equilibria
- Isoelectric and Isoionic pH
- 2.) Isoelectric point is the pH at which the
average charge of the polyprotic acid is 0 - isoelectric point A- H2A
Isoelectric point
37
Polyprotic Acid-Base Equilibria
- Isoelectric and Isoionic pH
- 3.) Example
- Determine isoelectric and isoionic pH for 0.10 M
alanine.
Solution For isoionic point
38
Polyprotic Acid-Base Equilibria
- Isoelectric and Isoionic pH
- 3.) Example
- Determine isoelectric and isoionic pH for 0.10 M
alanine.
Solution For isoelectric point
Isoelectric and isoionic points for polyprotic
acid are almost the same