5 Voltammetry - PowerPoint PPT Presentation
1 / 110
Title:
5 Voltammetry
Description:
A group of analytical methods based on determining current flow ... concentration of strong electrolytes (alkali metal salts) serves as supporting electrolyte ... – PowerPoint PPT presentation
Number of Views:1233
Avg rating:3.0/5.0
Title: 5 Voltammetry
1
5 Voltammetry
5-1 Basic principle of polarograpy
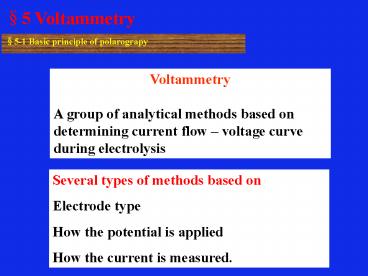
Voltammetry A group of analytical methods based
on determining current flow voltage curve
during electrolysis
Several types of methods based on Electrode
type How the potential is applied How the current
is measured.
2
(No Transcript)
3
When applied voltage reaches the decomposition
voltage of metal ion
Reduction reaction on cathode
Oxidation reaction on anode
U?- Ud iR
U? ? i
Where U? denotes applied potential, Ud is
decomposition potential, R is total resistance
of electrolysis circuit, i is current through
the circuit.
4
When following conditions are obeyed, U? i
graph is showed in following Figure
Lower current density during electrolysis
Adequately stirring to remove concentration
gradient
No quantitative relationship between current (or
potential) and concentration of interested ions
5
To find the analytical relationship between the
current and the concentration of sought-for ions
, following measurements are adopted
?MICROPLATINUM ELECTRODE Or DROPPING MERCURY
ELECTRODE ( in most common use) to insure
high current density
? NO STIRRING to insure enough high concentration
difference between the electrode surface and
the solution bulk
Then
6
When the current flowed for only a short period
through the electrode, ion concentration on the
surface of the electrode reduce suddenly. The
difference in ion concentrations between the
surface and the bulk solution is equivalent to an
electrochemical cell, which is called
concentration polarization(. thus, there is a
voltage that is equivalent to this concentration
change called concentration polarization
potential.
When a microplatinum electrode or dropping
mercury electrode is used and no stirring is
carried out, concentration polarization takes
place soon, following polarogram is obtained
7
(No Transcript)
8
When applied voltage does not reach reduction
voltage of metal ion, only a little current
flow, called residual current produced by
reduction of impurities and charging current, eg
AB in the Figure
Arriving on decomposition voltage, the electrode
reactions occur, the current rises rapidly with
increasing voltage, BC section in the figure
9
When it reaches C point voltage, due to
concentration polarization, the current arrives
at a limiting value and does not rise markedly,
called limiting current, consisting of residual
current and diffusion current
U ( ESCE -Ede ) i R
? i and R are very little in polarographic
electrolysis
U ESCE -Ede -Ede( vs. SCE)
To remove completely influence of iR, three
electrode system is usually used.
10
(No Transcript)
11
(No Transcript)
12
(No Transcript)
13
Polarography First voltammetric
technique Differs form hydrodynamic ?unstirred
(diffusion dominates) ?dropping Hg electrode
(DME) is used as a working electrode Current
varies as drop grows then falls off
14
(No Transcript)
15
In polarographic analysis, supporting electrolyte
is usually added to increase the
current-conductivity and eliminate migration
current. Usually relatively higher concentration
of strong electrolytes (alkali metal salts)
serves as supporting electrolyte
16
(No Transcript)
17
Advantages of DME (compared to planar
electrode) ? clean surface generated ? rapid
achievement of constant current during drop
growth ? remixing of solution when drop falls to
remove previous diffusion layer ? high Hg
overvoltage means even metals with high Ve E0
can be measured without H2 formation
Disadvantages of DME ? Hg easily oxidized,
limited use as anode(E lt 0.4V) 2Hg 2Cl-
-2e ?Hg2Cl2 ?nonfaradaic residual currents limit
detertion to gt 10-5 mol.L-1. ?cumbersome to
use(toxic mercury) ?sometimes produce current
maxima for unclear reasons( use maxima suppressor)
18
Sample Cells
19
(No Transcript)
20
(No Transcript)
21
Where Ce is Mn concentration on the electrode
surface, Ca is M concentration in amalgam on
electrode surface
22
Concentration polarization around mercury drop
23
When the applied voltage exceeds the
decomposition voltage, diffusion-controlled
current is expressed as
i K(C-Ce)
When the applied voltage gets more negative, Ce
?0, then id KC
Id reaches a limiting value proportional to ion
concentration C in bulk solution, and do not
changes with applied voltage longer
24
Ilkovic equation---diffusion current equation
In above equations, K is called Ilkovic constant,
it is expressed as follows
K 607 n D1/2m2/3t1/6 id 607nD1/2m2/3t1/6C
Concentration of electro-active analyte(mmol.L-1)
Drop time (sec)
Mercury mass flow rate(mg.sec-1)
Diffusion coefficient of electroactive analyte
in solution(cm2.sec-1)
From above equation, we can find that when
temperature, matrix solution and capillary
characteristic are kept constant, id is
proportional to C
Average limiting diffusion current denoting
average current on mercury drop from drop forming
to falling (mA)
Number of transferring electrons in electrode
reaction(e/mol)
25
(No Transcript)
26
(No Transcript)
27
How it works? ? The applied voltage is gradually
increased, typically by going to a more positive(
more negative decomposing potential) ? A small
residual current is observed. ? When the voltage
becomes great enough, reduction occurs at the
analytical electrode causing a current. ? The
electrode is rapidly saturated so current
production is limited based on diffusion of the
analyte to the small electrode.
28
How it works ? The reduced species alters the
surface of the mercury electrode. To prevent
problems, the mercury surface is renewed by
knocking off a drop providing a fresh
surface. This results in an oscillation of the
data as it is collected.
29
Polarographically quantitative analytical methods
?Direct comparison method
30
(No Transcript)
31
?Calibration curve method
As shown in right graph
32
?Standard addition method
When only one sample is analysized, this method
can be employed. At first measuring the
polarographic wave height hx of an unknown
solution with a volume of V, then a little amount
of the standard solution of concentration CS with
a volume of VS is added and the wave height of
the mixture solution is measured. As we know,
following equations are vivid
33
(No Transcript)
34
Quantitative Analysis From Ilkovic equation Id
KC Usually use method of standard
Additions(with uL additions, no volume correction
needed) Detection Limits 10-5
10-6mol.L-1 Resolution DE1/2 0.2V(not very
good) How do the DL and Resolution be improved?
35
5-3 Halfwave potential polarographic
qualitative analysis
CAe concentration of A on mercury drop surface CA
concentration of A in bulk solution CBe
concentration of B the electrode surface
if B soluble, CBe concentration of B in the
solution near the electrode if B forms
amalgam, then it means B concentration in
amalgam if B is metal element and
insoluble in mercury, then it equals to 1 CB
equals to zero, the concentration of B in bulk
solution
36
Nernst equation is still vivid here
Where -id kACA
-i kA(CA- CAe)
CAe may be calculated according to above eqs.
37
On the other hand, according Faradaic law, during
electrolysis, concentration of reduction
production B is proportional to current flow,
that is,
CBe ? - i
Hence
38
?
When i ½ id , log term in above equation is
equal to zero, corresponding potential is called
halfwave potential E1/2
()
?E1/2 independent on the concentration ?basis of
qualitative analysis
39
Because cathode current is in more common use,
equation () is rewriting as
For three types of polarographic waves mentioned
above, E1/2 have a same expression as formula()
40
(No Transcript)
41
(No Transcript)
42
Why using E1/2 but not Edec as a qualitative
analysis basis?
43
(No Transcript)
44
(No Transcript)
45
(No Transcript)
46
(No Transcript)
47
(No Transcript)
48
(No Transcript)
49
5-4 Interference currents and their removing
methods
? residual current
redox reactions of impurities in solution
charging of Hg drop (non-faradaic current /
non-redox current) Charging current formation
is shown in the figures next page
50
(No Transcript)
51
(No Transcript)
52
?
53
? Migration current
In polarographic analysis, you must remove
convection migration of the sought-for ions in
the solution Why?
Migration current The current produced by static
attraction of the electrode to sought-for ions
54
How to remove them?
Hold the solution stil to eliminate
convection Add supporting electrolyte to remove
migration Supporting electrolyte strong
electrolyte, inert (electro-inactive), with a
concentration 100 times than sought-for ion
55
?Maximum (or malformed peak )
Measurements removing current maxima
Add maximum suppresor, usually surfactant, eg.
56
? Oxygen wave
57
(No Transcript)
58
(No Transcript)
59
5-5 Characteristics and disadvantages of
polarographic analysis
- ?advantages
Relatively high sensitivity, kinear range 10-2
10-4mol.L-1 Relative error 2, as good as
that of spectrophotometry Simultaneously
determine 4 5 analytes at one time under
appropriate condition A little amount of test
sample needed rapid analysis Excellent
reproductivity (??????,??????????) Wide
application field (metal ion, metal complex,
anion or organic compounds etc that is
electro-active)
60
?Disadvantages
poor sensitivity prewave interference
poor resolution, DE1/2 100mV for practical
separation of two peaks
61
5-6 Polarographic Catalytic Wave
62
A is called catalyst polarographic current
is called catalytic current, i1?A chemical
reaction is its velocity-determining step X
must have high over-potential on the electrode
to insure catalytic circle
63
For example
64
When no adsorption exists, catalytic wave and
classical polarographic wave have same shape,
catalytic current is expressed as follows
i1 0.51nF D 1/2 m 2/3 t 2/3 k 1/2 Cx1/2 CA
Concentration of X and A in solution
Limiting catalytic current
Rate constant
65
Difference between catalytic wave and diffusion
wave
For polarographic catalytic wave, the current is
independent on height of mercury column
i1 ? m 2/3t 2/3 ? h 2/3 h -2/3 ? 1
But for diffusion-controlled current
id ? m 2/3t 1/6 ? h 2/3 h -1/6 ? h1/2
Temperature coefficients 1 2 / ? for id 4
5 and more / ? i1
????(??)
66
5-7 Single-sweep polarography
(Oscillographic polarography,
Classical polarography Slow direct current
voltage scanning rate 0.2V/min, some 100
drops of mercury per scan Big residual current
Single-wseep polarography Rapid scanning
rate 0.25V/sec, one drop of mercury per scan
Little residual current Oscillographic
polarograph
67
ip and Ep are respectively peak current and peak
voltage U is toothed wave voltage(?????)
68
For reversible electrode reaction, diffusion
current equation of single-sweep polarography is
expressed as follows
ip 2.69105n3/2 D1/2 u 1/2 AC
u is scan rate of voltage(V.s-1), A is electrode
area (cm2)
Relationship between potential at peak Ep and
halfwave potential E1/2
For reduction wave, Ep is -28/n mV more negative
than corresponding E1/2, and for oxidative wave,
28/n mV more positive than E1/2
69
?Advantages of single-sweep polarography
- Sensitivity DL 10-7mol.L-1, 2-3 magnitude
order higher than classical polarography - More precise, measuring peak height, but not wave
height - Simple, rapid
- Higher resolution, peak separation of 35 50mV
resolved - Little prewave interference, 5s interval time
before scanning - Little oxygen wave interference, in-reversible
electrode reaction shows very weak or even no wave
70
BACK
71
(No Transcript)
72
(No Transcript)
73
Back
74
(No Transcript)
75
? Cyclic voltammetry
76
The peak potential difference between cathodal
peak and anodic peak is
,DEp gt 56.5/n(mV), ipa / ipc lt 1,
77
(No Transcript)
78
(No Transcript)
79
(No Transcript)
80
(No Transcript)
81
(No Transcript)
82
5-8 Squar-wave polarography
83
?
84
5-9 Pulse polarography
85
(No Transcript)
86
(No Transcript)
87
(No Transcript)
88
(No Transcript)
89
???????????????????
90
5-10 Stripping voltammetry
91
(No Transcript)
92
(No Transcript)
93
(No Transcript)
94
?????????????????????????
95
Electrodes in stripping voltammetry
?Hung mercury electrode ? glass carbon electrode
mercury plating(???????????)
Problems
Compared with other polarographic methods, how
about the sensitivity of sripping
voltammetry? what advantages do hung mercury
electrode and mercury membrane electrode
have ?
96
(No Transcript)
97
(No Transcript)
98
(No Transcript)
99
(No Transcript)
100
(No Transcript)
101
5-11 ?????????(Amperometric titration with
single indicating electrode, polarographic
titration)
??????????????????????????????????????????????????
??,????????????.???????????????????,???????.
Amperometric titration keeping potential
constant Potentiometric titration keeping
current constant or at null-current
102
a analyte is electroactive (able to be reduced
on electrode), but the titrant is not b
analyte and titrant are all able to be reduced on
the polar electrode
103
Electrodes
Reference electrode calomel electrode,
mercury pool
electrode Working electrode dropping mercury
electrode,
solid microelectrode
Advantages
Solid microelectrode can be used wide
linear range. 0.1 10-4mol.L-1 Using a
electrode with a big area, DL can be down to
10-6 mol.L-1 Back titration can increase
upper limit of determination some
electro-inactive materials can also be measured
using electro-active titrant.
104
Example
105
5-12 (Dead-stop end point titration)
106
Ce4 Fe2 Ce3 Fe3
?
107
Titration curve of dead-stop end point method
?With reversible system titrating reversible one,
curve ABCE in the left figure. Eg. Ce(IV)?Fe3 ?
With irreversible system titrating reversible
one, curve ABCD in the left figure, eg. S2O32-
?I2 ? With reversible system titrating
irreversible, curve ABC in the lower left side.
Eg. I2 ? S2O32-(???)
108
(potentiometric titration with double
indicating electrodes)
?potentiometric titration includes two kinds
single-indicating electrode
double-indicating electrode
?
109
(No Transcript)
110
????? ??????,?????pH?????????