ELECTROCHEMISTRY Chapter 21 - PowerPoint PPT Presentation
1 / 31
Title:
ELECTROCHEMISTRY Chapter 21
Description:
Electrons are 'driven' from anode to cathode by an electromotive force or emf. ... 'distance' from 'top' half-reaction (cathode) to 'bottom' half-reaction (anode) ... – PowerPoint PPT presentation
Number of Views:117
Avg rating:3.0/5.0
Title: ELECTROCHEMISTRY Chapter 21
1
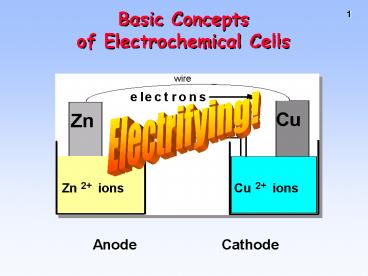
Basic Concepts of Electrochemical Cells
Electrifying!
Anode
Cathode
2
CHEMICAL CHANGE ---gtELECTRIC CURRENT
With time, Cu plates out onto Zn metal strip, and
Zn strip disappears.
- Zn is oxidized and is the reducing agent Zn(s)
---gt Zn2(aq) 2e- - Cu2 is reduced and is the oxidizing
agentCu2(aq) 2e- ---gt Cu(s)
3
CHEMICAL CHANGE ---gtELECTRIC CURRENT
- Oxidation Zn(s) ---gt Zn2(aq) 2e-
- Reduction Cu2(aq) 2e- ---gt Cu(s)
- --------------------------------------------------
------ - Cu2(aq) Zn(s) ---gt Zn2(aq) Cu(s)
4
CHEMICAL CHANGE ---gtELECTRIC CURRENT
- To obtain a useful current, we separate the
oxidizing and reducing agents so that electron
transfer occurs thru an external wire.
This is accomplished in a GALVANIC or VOLTAIC
cell. A group of such cells is called a battery.
5
Zn --gt Zn2 2e-
Cu2 2e- --gt Cu
Oxidation Anode Negative
Reduction Cathode Positive
lt--Anions Cations--gt
- Electrons travel thru external wire.
- Salt bridge allows anions and cations to move
between electrode compartments.
6
The CuCu2 and AgAg Cell
Electrons move from anode to cathode in the
wire. Anions cations move thru the salt bridge.
7
Anode, site of oxidation, negative
Cathode, site of reduction, positive
8
CELL POTENTIAL, E
Zn and Zn2, anode
Cu and Cu2, cathode
- Electrons are driven from anode to cathode by
an electromotive force or emf. - For Zn/Cu cell, this is indicated by a voltage of
1.10 V at 25 C and when Zn2 and Cu2 1.0
M.
9
CELL POTENTIAL, E
- For Zn/Cu cell, potential is 1.10 V at 25 C and
when Zn2 and Cu2 1.0 M. - This is the STANDARD CELL POTENTIAL, Eo
- a quantitative measure of the tendency of
reactants to proceed to products when all are in
their standard states at 25 C. - This means pure solids or in solution at a
concentration of 1M!!!!
10
Calculating Cell Voltage
- Balanced half-reactions can be added together to
get overall, balanced equation.
Zn(s) ---gt Zn2(aq) 2e- Cu2(aq) 2e-
---gt Cu(s) ---------------------------------------
----- Cu2(aq) Zn(s) ---gt Zn2(aq) Cu(s)
- If we know Eo for each half-reaction, we could
get Eo for net reaction. - Lets revisit my haiku!
11
Oxidation Haiku!
- Lost an electron
- But now feeling positive
- Oxidized is cool!
- What is that? You want a reduction Haiku?
12
Reduction Haiku!!!
- Gained some electrons
- Gave me a negative mood!
- Now I can say Ger!
- Thank you Enjoy the buffet Dont eat the
chemicals or furniture kids!
13
CELL POTENTIALS, Eo
- Cant measure 1/2 reaction Eo directly.
Therefore, measure it relative to a STANDARD
HYDROGEN CELL, SHE.
2 H(aq, 1 M) 2e- lt----gt H2(g, 1 atm)
Eo 0.0 V
14
Zn/Zn2 half-cell hooked to a SHE. Eo for the
cell 0.76 V
Supplier of electrons
Acceptor of electrons
2 H 2e- --gt H2 Reduction Cathode
Zn --gt Zn2 2e- Oxidation Anode
15
Reduction of H by Zn
Figure 20.10
16
Overall reaction is reduction of H by Zn
metal. Zn(s) 2 H (aq) --gt Zn2 H2(g) Eo
0.76 V Therefore, Eo for Zn ---gt Zn2 (aq)
2e- is 0.76 V Zn is a (better) (poorer) reducing
agent than H2.
17
Cu/Cu2 and H2/H Cell
- Eo 0.34 V
Positive
Negative
Acceptor of electrons
Supplier of electrons
Cu2 2e- --gt Cu Reduction Cathode
H2 --gt 2 H 2e- Oxidation Anode
18
Cu/Cu2 and H2/H Cell
- Overall reaction is reduction of Cu2 by H2 gas.
- Cu2 (aq) H2(g) ---gt Cu(s) 2 H(aq)
- Measured Eo 0.34 V
- Therefore, Eo for Cu2 2e- ---gt Cu is
0.34 V
19
Zn/Cu Electrochemical Cell
Anode, negative, source of electrons
Cathode, positive, sink for electrons
- Zn(s) ---gt Zn2(aq) 2e- Eo 0.76 V
- Cu2(aq) 2e- ---gt Cu(s) Eo 0.34 V
- --------------------------------------------------
------------- - Cu2(aq) Zn(s) ---gt Zn2(aq) Cu(s)
- Eo (calcd) 1.10 V
20
Yes It is finally time for a DEMO!!
- Do you feel like bridging that salt?
21
TABLE OF STANDARD REDUCTION POTENTIALS
2
22
Potential Ladder for Reduction Half-Reactions
Figure 20.11
23
Table 21.1 Page 970
24
Standard Redox Potentials, Eo
Any substance on the right will reduce any
substance higher than it on the left.
Northwest-southeast rule product-favored
reactions occur between reducing agent at
southeast corner (anode) and oxidizing agent at
northwest corner (cathode).
25
Standard Redox Potentials, Eo
- Any substance on the right will reduce any
substance higher than it on the left. - Zn can reduce H and Cu2.
- H2 can reduce Cu2 but not Zn2
- Cu cannot reduce H or Zn2.
26
Using Standard Potentials, EoTable 20.1
- In which direction do the following reactions go?
- Cu(s) 2 Ag(aq) ---gt Cu2(aq) 2 Ag(s)
- 2 Fe2(aq) Sn2(aq) ---gt 2 Fe3(aq) Sn(s)
- What is Eonet for the overall reaction?
27
Standard Redox Potentials, Eo
Enet distance from top half-reaction
(cathode) to bottom half-reaction (anode) Enet
Ecathode - Eanode
Eonet for Cu/Ag reaction 0.46 V
28
Eo for a Voltaic Cell
Cd --gt Cd2 2e- or Cd2 2e- --gt Cd
Fe --gt Fe2 2e- or Fe2 2e- --gt Fe
All ingredients are present. Which way does
reaction proceed?
29
Eo for a Voltaic Cell
- From the table, you see
- Fe is a better reducing agent than Cd
- Cd2 is a better oxidizing agent than Fe2
Overall reaction Fe Cd2 ---gt Cd
Fe2 Eo Ecathode - Eanode (-0.40 V) -
(-0.44 V) 0.04 V
30
More About Calculating Cell Voltage
- Assume I- ion can reduce water.
2 H2O 2e- ---gt H2 2 OH-
Cathode 2 I- ---gt I2 2e-
Anode --------------------------------------------
----- 2 I- 2 H2O --gt I2 2 OH- H2
Assuming reaction occurs as written, Enet
Ecathode - Eanode (-0.828 V) - (0.535 V)
-1.363 V Minus E means rxn. occurs in opposite
direction
31
If you have reached this far, you need a break!