Ozone - PowerPoint PPT Presentation
1 / 57
Title:
Ozone
Description:
Processing to glucose from non-cellulosic material is much easier (amylase) ... Processing from cellulosic ethanol is difficult: ... – PowerPoint PPT presentation
Number of Views:67
Avg rating:3.0/5.0
Title: Ozone
1
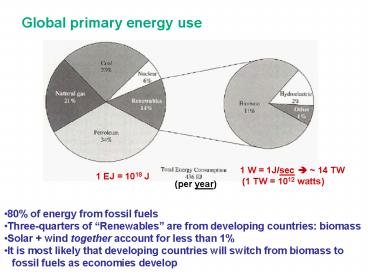
Global primary energy use
1 W 1J/sec ? 14 TW (1 TW 1012 watts)
1 EJ 1018 J
(per year)
- 80 of energy from fossil fuels
- Three-quarters of Renewables are from
developing countries biomass - Solar wind together account for less than 1
- It is most likely that developing countries will
switch from biomass to - fossil fuels as economies develop
2
Growth in Energy Efficiency
Annual global growth rate in use of commercial
energy 2 Total energy/ year 400 x 1018 J
400 exajoules 1EJ 1018 J 100 EJ/year by
United States Over time in industrialized
countries the amount of energy consumed to
generate a unit of economic growth decreases
panel (a) Carbon intensity ratio of
CO2-equivalent emissions per unit of GDP
panel (b)
2003 US proposal in lieu of signing Kyoto accord
well reduce carbon intensity 18 by 2012! BUT
a 14 reduction would have been achieved without
doing anything new
3
Expected future growth in energy consumption
much more in the developing countries Energy
efficiency in developed countries holds down
the growth of energy consumption
4
(No Transcript)
5
Stabilization Wedges Solving the Climate
Problem
BAU business as usual 1.5/yr avg growth
rate in CO2 emissions from the period 1975-2005
Present carbon emissions level 7
GtC/year Assertion we can solve the carbon
and climate problem in the next 50 years by
applying known technologies BAU reaches 14
GtC/yr. by 2050
Sci. Am. September 2006, p. 50 also see suppl.
material for Science 305, 968
6
Stabilization Wedges Solving the Climate
Problem
BAU business as usual 1.5/yr avg growth
rate in CO2 emissions from the period 1975-2005
Present carbon emissions level 7 GtC/year A
wedge starts at zero and reaches 1 GtC/yr.
by 2056 Total additional carbon in the BAU is
175 GtC from 2006-2056 Each wedge removes a
total of 25 GtC by 2056 Seven wedges applied to
reach 1 GtC/yr saved each by 2056 175 GtC
why a wedge?
Sci. Am. September 2006, p. 50 also see suppl.
material for Science 305, 968
7
Stabilization Wedges Solving the Climate
Problem
BAU business as usual 1.5/yr avg growth
rate in CO2 emissions from the period 1975-2005
Of course, to achieve stabilization at any
level requires that ultimately the emissions
drop to zero This scenario if followed to
2056 would leave us at about 500 ppm
CO2 Scenario assumes 2 growth in energy con-
sumption/year Uncertainties population, C
sources/sinks
Sci. Am. September 2006, p. 50 also see suppl.
material for Science 305, 968
8
15 wedges suggested
- General areas in
- which to achieve
- a wedge
- Energy efficiency
- Decarbonization of
- energy supply
- Shift fossil fuels
- CCS
- Alternative Energy
- Agriculture/Forestry
9
Agriculture/Forestry Improving natural sinks
Two wedges achievable from Reducing tropical
deforestation Eliminate primary clearcutting
0.5 wedge Reforesting already-cut areas 0.5
wedge Establish new plantations on non-forested
land 0.5 wedge Improve crop tilling practices
carbon lost by increased aeration
comprehensive conservation tillage 0.5 1.0
wedge Issues preservation of biodiversity decre
ase likelihood of massive rainforest loss as T
increases need for land dedication to
agriculture verification and reversibility of
crop tillage practices
10
Energy efficiency and conservation
Four wedges achievable from Increased auto fuel
economy (2 x 109 cars) 30 mpg average to 60 mpg
average 1 wedge reduce average mileage from
10K to 5K/year 1wedge Cut electricity use in
residential and commercial settings by 25 1
wedge Increase efficiency at coal-fired
electricity plants from 40 to 60 Plants of 1
GW capacity or larger, with 2x increase in coal
use Issues less tangible generally arise from
many small innovations design of urban areas and
mass transportation need for Government
incentives
11
Decarbonizing Energy Supply
Nine wedges achievable from Switch from
coal-fired to gas-fired power generation 1
wedge Carbon emissions are roughly half at
natural gas plants Switch 4x as much coal to
gas, as there is gas presently used Implement
CCS (carbon capture and storage) 3
wedges Prevents 90 of carbon emissions, and
sequesters underground Install at conventional
coal or gas power plant 1 wedge Install at
H2-generation plants (H2 from coal, gas) 1
wedge Install at coal ? synfuels plants 1
wedge Alternative energies (replace coal
equivalent of energy) 3 wedges Add 2x current
capacity (700 GW) of nuclear fission 1
wedge Add 50x current capacity wind power (2
million at 1 MW) 1 wedge Add 700x current
capacity (2000 GW) solar power 1
wedge Alternative energies (replace gasoline
equivalent of energy) 2 wedges Add 100x
current capacity wind power to make H2 for cars
1 wedge Add 100x current US ethanol production
(1/6 world cropland) 1 wedge
12
(No Transcript)
13
Origins of fossil fuels
Carbon cycling in the biosphere CO2 H2O hv ?
CH2O O2 CH2O O2 ? CO2 H2O Not a closed
loop some plant and animal matter is buried,
accumulates in deposits at high T and P ? coal,
oil and gas
14
Coal forming forest/swamp
Coal the remains of plant matter from very
large woody swamps -Plant material is mainly
cellulose and lignin
Peat
Cellulose is degraded by aerobic bacteria
Lignite
Bituminous
Anthracite
15
Coal forming forest/swamp
Coal the remains of plant matter from very
large woody swamps -Plant material is mainly
cellulose and lignin
Peat
Lignin resists biological degradation
Lignite
Bituminous
The lignin accumulates under water, and over
time compacts into peat Burial due to geological
forces, compacting, high T and P ? coal.
(further compression may yield graphite a form
of pure carbon)
Anthracite
16
Coal forming forest/swamp
Types of coal Lignite softest, 37 moisture,
only 30 fixed carbon. Heating value low 16
kJ/gram Bituminous various grades, 2-3 water
and 55-65 fixed carbon. Heating value 30
kJ/gram Anthracite 4 moisture, gt80 fixed
carbon Heating value 30 kJ/gram
Peat
Lignite
Bituminous
Anthracite
17
Where coal is found in the US and around the
world
Resource estimated natural occurrence of a
material Reserve subset of the resource
available for current exploitation
- World coal resources
- 200,000 EJ (7 trillion tons)
- World coal reserves
- 21000 EJ
- Current use 100 EJ/year
- Remaining reserve 210 years
- From 2000 UNDP World Energy
- Assessment
- US NRC estimates much
- lower than previously predicted
- (Science 323, 1420-21 (2009))
18
Geological conditions for accumulation of oil and
gas
- Petroleum and natural gas are of marine origin
- Anaerobic bacterial degradation of biological
matter releases most of the - O and N, but hydrocarbon-based lipids survive
- Sediment becomes deeply buried over time (high T
and P) ? oil/gas - Forms in specific geological substructures,
trapped in porous rock from - which water is squeezed out as the sediment
compacts
19
Composition of Petroleum -a complex mixture of
hydrocarbons linear (CnH2n2), branched, and
cyclic -crude oil is separated in a distillation
tower into gasoline (C5-C12) kerosene/jet
fuel diesel oil and home heating
fuel steam-generating industrial
fuel lubricating oil (C19-C35) wax
Increasing number Cs and MW, increased boiling
range
-Petroleum also contains some aromatic compounds
(BTX component)
-reactors at oil refineries also operate on the
mixture to change the composition eg,
cracking of C18-C20 to C8-C10 range for
gasoline (high T catalyst)
20
- Refined gasoline still has poor burning
properties - in internal combustion engines spontaneous
ignition before full - compression in a cylinder knocking
- Highly branched alkanes have excellent burning
properties, so are added in
Octane number ability of gasoline to generate
power w/o engine knocking isooctane 100
n-heptane 0 refined gasoline 50
Other additives that reduced knocking but had
issues Pb(CH3)4 tetramethyl lead now
banned MTBE Octane 116 - water-soluble,
resistant to biological degradation, contaminated
well water banned for use in gasoline by CA in
2003
21
Unconventional Oil Oil Shale Deposits
Recovery of shale oil requires energy-intensive
processing kerogen heat (ca. 480C) gt
hydrocarbons carbonaceous residue cooling of
hydrocarbons gt shale oil yields ca. 38 liters
of oil/ton Alternative mine, transport, and
burn it directly to produce electricity Size of
resource estimated at 3 trillion barrels (3x
conventional oil) Energy Policy Act 2005
permits commercial oil shale leases on Federal
land
22
Canadian Oil Sands
An example of unconventional oil
BEFORE
- Steam injection to gasify
- Methane burned to make steam
- EROEI 32
- North American methane
- production in decline as well
AFTER
23
Geological conditions for accumulation of oil and
gas
- Natural gas deposits often found together with
oil in a higher strata - Now represents 25 of the US energy budget
- Cleaner than coal or oil because of lower CH
ratio - Need high P or low T (or both) to use in
transport so far only trucks/buses
24
- LNG- Liquefied natural gas
- Methane is drilled, refined, liquefied at
- a remote site
- The LNG is loaded on double-hulled
- refrigerated tankers and shipped
- The LNG is expanded offshore or onshore,
- and piped to interface with a local gas
- network
1.
3.
2.
25
- Proposed West Coast
- LNG terminals for
- gas shipped from overseas
- BHP Billington proposal for
- offshore Oxnard at
- Cabrillo Port
- Authorization denied both by
- CA State Lands Commission
- (lease for pipeline under CA
- shoreline)
- AND
- CA Coastal Commission
- (April 2007)
26
(No Transcript)
27
How much oil is there?
Hubberts peak geologist M. King Hubbert, in
1950s, correctly predicted a US oil production
peak in 1970
28
USA Lower 48 Oil Discovery and Production
Oil depletion in the US shows a 42 year gap
between peak discovery and peak production. No
new large fields found since early 1980s. As
goes the US, so goes the world?
29
Worldwide growing gapbetween oil discovery and
production
Green vertical bars depict years where
Discoveries exceeded Production. The red bars
show years where Discoveries were less than
Production Current ratio 4 barrels consumed
for every new barrel of oil found
30
World Conventional Oil
Global oil production has been essentially flat
since about 2000 The world is on a plateau
where the US alone was in about 1970
31
(No Transcript)
32
Total Barrels
Alternative view CERA Cambridge Energy
Research Associates Claim 3x greater reserves
than most other analysts Optimism that much
more of the resource will become a reserve
33
- Its tough to make predictions, especially about
the future - -Yogi Berra
- Field-by-field data often unavailable, especially
from the Middle East - Clear measured declines (5-20/year) in many
areas, including - Alaskan North Slope, Mexico (Cantarell
field), North Sea - Some offsetting gains from new fields coming on
line - Angola, Brazil, Middle East (?)
- Saudi Arabia promises increased supplies but can
they deliver? - Improved oil recovery technology?
- Ramp-up from expensive unconventional oil, as
prices continue to rise - Resources reassigned as reserves
Simmons Company International
34
A SUMMARY OF PREDICTIONS FROM THE EXPERTS
35
The Hydrocarbon Age
36
Some statistics on conventional oil Total world
conventional oil reserves in 1780 2 trillion
barrels (2 x 1012) 1780 Watt develops the steam
engine Industrial Revolution starts Total world
conventional oil reserves remaining (2007) 1
trillion barrels Yearly rate of world oil
consumption 31 billion barrels (31 x 109) Daily
rate 85 million barrels burned per
day Consumption in the United States US burns
25 of all the oil (and all energy use) in the
world US of total world population (6.7
billion) 4.6 Should we drill in the Arctic
National Wildlife Refuge? Alaskan oil yields
most optimistic estimates are for 1 million
barrels per day starting in about 10
years Present US consumption 18 million barrels
per day Potential net gain 6 increase in oil
availability Potential net loss Alaskan
environmental damage
37
(No Transcript)
38
Coal-Derived Fuels
-make the coal cleaner by increasing the H/C
ratio Hydrogasification C 2 H2 ? CH4 T
800C DH -75 kJ/mol At high T, reaction
proceeds in the reverse direction Methanation of
CO CO 3 H2 ? CH4 H2O T 400C Ni
catalyst DH -206 kJ/mol Production of liquid
fuels from CO (Fischer-Tropsch chemistry) nCO
(2n 1)H2 ? CnH2n2 nH2O Production of
methanol from CO CO 2 H2 ? CH3OH Where do
the CO and H2 come from?
39
Coal-Derived Fuels
Production of CO and H2 from coal Steam
reforming C H2O ? CO H2 T 900C DH
131 kJ/mol Equimolar CO and H2 are
produced Water-gas shift reaction CO H2O ?
CO2 H2 DH -41 kJ/mol Produces additional
hydrogen gt21 mole ratio of H2CO needed to
produce liquid fuels This chemistry was used by
Germany in WWII when the Allies cut off oil
shipments into the country.
40
Coal-Derived Fuels
- Combining these reactions
- CO 3 H2 ? CH4 H2O (methanation of CO DH
-206.3 kJ/mol) - 2C 2H2O ? 2CO 2H2 (steam reforming DH
131.4 kJ/mol)) - CO H2O ? CO2 H2 (water-gas shift
reaction DH -41.4 kJ/mol) - 2C 2 H2O ? CO2 CH4 (combining reactions)
- Overall DH 15 kJ/mol
- ? all the heating value of coal can be
transferred to CH4 - with only 15 kJ/mol energy expenditure (in
theory) - Unfortunately the reactions are poorly matched
heat derived - from methanation cant drive steam reforming
because the latter - needs an extremely high temperature
- So steam reforming is driven by burning more coal
- Energy efficiency of the overall process is
lowered - Greenhouse effect is larger than producing the
same energy from coal alone
41
Production of hydrogen (and food)
- Can be accomplished from oil and gas as well as
coal - CH4 2 H2O ? 4 H2 CO2 (hydrogen gas from
methane reforming) - This is the major route to H2 today
- Fossil fuel production of H2 is used to produce
ammonia (Haber process) - N2 3 H2 ? 2 NH3 from thin air
- Then NH3 2 O2 ? HNO3 H2O
- NH3 HNO3 ? NH4NO3
- to produce fertilizers
- Food production at levels needed to sustain
current population is - presently almost fully dependent on nonrenewable
fossil fuel
42
Emissions in the United States
CO2 Emissions From Coal-Fired Electricity
Generation 1897 billion tons
31.7
Other Emissions 3675 billion tons
CCS is a viable strategy in the stationary
power plants, not for the mobile sources of
CO2
CO2 Emissions From Other Electricity
Generation 416 billion tons
U.S. Total CO2 Emissions 5,988 billion tons
(2004)
43
Capture and Geologic Storage of CO2 Avoids
Emissions
- CO2 is scrubbed from the smoke stack emissions
- CO2 is injected deep underground
A Four Step Process
Capture
Compression
Underground Injection
Pipeline Transport
44
Options for CO2 Capture
- Post-combustion
- Established technology
- Pre-combustion
- Established technology for other applications
- Not demonstrated for power production
- Oxygen combustion
- Not demonstrated for power production
Burning directly in oxygen produces only CO2
and water but too hot for available materials
45
Options for CO2 Capture
- Post-combustion
- Conventional pulverized coal plant
- Burn coal in air
- Exhaust is mostly N2 and 15 CO2
- Scrubbing of CO2 uses amines
- R2NH (l) CO2 ? R2NCOO- H
- The CO2 enters the liquid phase
- The amine liquid is then separated and heated to
release the concentrated CO2 for capture - Requires retrofitting of existing plants
46
Options for CO2 Capture
- Pre-combustion
- IGCC integrated gasification
- combined cycle
- Gasify the coal first produces
- syngas CO H2
- Water shift reaction to generate
- CO2
- Remove SO2, other impurities
- Remove CO2 with amine reaction
- Burn very clean H2
47
Where to store the carbon? (at least several
GtC/yr) -deep ocean burial -very deep
aquifers -depleted oil/gas reservoirs
Dissolving CO2 in seawater CO2 (g) H2O ?
H2CO3 ? H HCO3- Ocean acidic means
depositing CO2 sufficiently offshore and deep
enough so that a CO2-water clathrate might form
Ocean neutral is better react CO2 with CaCO3
or CaSiO3 CO2 (g) H2O CaCO3 (s) ?
Ca(HCO3)2 (aq) --traps CO2 so it will not
ultimately escape (if at ocean bottom)
48
Options for Geological Storage
- Oil and gas fields
- Depleted
- EOR, EGR
- Saline formations
- Unminable coal-seams
From IPCC Special Report
49
What Keeps the CO2 Underground?
Ground Surface
- Injected at depths of 1 km or deeper into rocks
with tiny pore spaces - Primary trapping
- Beneath seals of low permeability rocks
- Secondary trapping
- CO2 dissolves in water
- CO2 is trapped by capillary forces
- CO2 converts to solid minerals
Sand
Shale
Sandstone
Shale
Sandstone
Shale (seal)
1/10 inch
Storage security increases over time due to
secondary trapping mechanisms.
Sandstone (storage formation)
50
Multiple Lines of Evidence Indicate Storage Can
Be Secure and Effective
- Natural analogues
- Oil and gas reservoirs
- CO2 formations
- Industrial analogues
- CO2 EOR
- Natural gas storage
- Liquid waste disposal
- Existing projects
- Sleipner, Off-shore Norway
- Weyburn, Canada
- In Salah, Algeria
470 natural gas storage facilities in the U.S.
20 to 30 Mt/yr are injected for CO2-EOR
51
Capacity of Storage Formations
a. Estimates would be 25 larger if undiscovered
reserves were included.
From IPCC Special Report
Available evidence suggests that worldwide, it
is likely that there is a technical potential of
at least about 2,000 GtCO2 (545 GtC) of storage
capacity in geological formations.
52
(No Transcript)
53
Biomass as renewable energy
Carbon cycling in the biosphere CO2 H2O hv ?
CH2O O2 CH2O O2 ? CO2 H2O Biomass is
burned, but no net CO2 is introduced into the
atmosphere.
Energy density is lower than fossil fuel
- Sources
- agricultural waste
- explicit energy crops
54
Production of ethanol from biomass
Ethanol production is based on (anaerobic)
fermentation of sugars Ethanol is mixed with
gasoline (improve octane) or used as E85 Very
successful in Brazil (sugar cane)
- Ethanol fermentation starts from pyruvate CO2
production - makes the resulting mixture carbonated (beer),
or causes - dough to rise (bakers yeast).
- Pyruvate is the product of a 10-step enzyme
pathway that breaks down glucose - (glycolysis)
55
Production of ethanol from biomass
- Crops are harvested and processed to yield
glucose or other sugars that - can be fermented to ethanol.
- Processing to glucose from non-cellulosic
material is much easier (amylase) - Cellulase enzymes are found only in certain fungi
and bacteria and are - much harder to adapt in industrial processes
56
- Efficiency of energy conversion from corn
- ethanol is extremely poor. Why?
- Fermentation plant requires fossil fuel input
- Corn harvesting and ethanol transport
- Fertilizers! Ultimately depend on CH4
- Corn ethanol interest in the US is driven
- by politics, not science
57
Processing from cellulosic ethanol is
difficult Cellulose is covered in hemicellulose
requiring acid treatment Hemicellulose lignin
5-carbon sugar polymer Once uncovered
cellulose has to be processed with cellulases
? glucose Xylose requires separate fermentation
from glucose
This processing currently limits noncellulosic
ethanol production to pilot-scale Dedication of
land to biomass production also is limiting
lignin
xylose