P1259070314npdoV - PowerPoint PPT Presentation
1 / 9
Title:
P1259070314npdoV
Description:
1. One mole of an ideal monoatomic gas is put through the reversible cycle shown ... Find the work, heat, change in Energy, and change in Enthalpy, for each stage ... – PowerPoint PPT presentation
Number of Views:40
Avg rating:3.0/5.0
Title: P1259070314npdoV
1
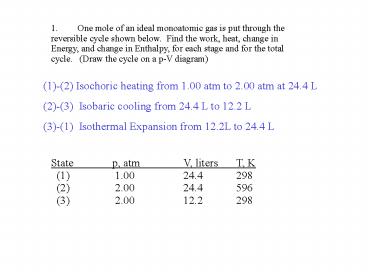
1. One mole of an ideal monoatomic gas is put
through the reversible cycle shown below. Find
the work, heat, change in Energy, and change in
Enthalpy, for each stage and for the total cycle.
(Draw the cycle on a p-V diagram)
(1)-(2) Isochoric heating from 1.00 atm to 2.00
atm at 24.4 L (2)-(3) Isobaric cooling from 24.4
L to 12.2 L (3)-(1) Isothermal Expansion from
12.2L to 24.4 L
State p, atm V, liters T, K (1)
1.00 24.4 298 (2) 2.00 24.4 596
(3) 2.00 12.2 298
2
One mole of an ideal monoatomic gas CV (3/2)R
Cp (5/2)R is put through the reversible
cycle shown below. popp p Find the work,
heat, change in Energy, and change in
Enthalpy for each stage and for the total
cycle. ?Hnet 0 ?Unet 0 Cycle!
(1)-(2) Isochoric heating from 1.00 atm to 2.00
atm at 24.4 L
Isochoric w0 Ti 298 Tf 596 ?T
298 ?U CV?T 3.72 kJ ?H Cp?T 6.19 kJ qV
?U 3.72 kJ
3
(2)-(3) Isobaric cooling from 24.4 L to 12.2 L
Isobaric q ?H Cp?T Ti 596 Tf 298
?T -298 ?U CV?T -3.72 kJ ?H Cp?T
-6.19 kJ qp ?H -6.19 kJ w -popp?V -2
(24.4-12.2) 24.4 l.atm w (8.314 J.mol-1.K
-1 / 0.08205 l.atm mol-1.K -1) 22.4 l.atm w
2.47 kJ ?U - q
4
(3)-(1) Isothermal Expansion from 12.2L to 24.4 L
Isothermal ?H 0 ?U 0 w -?popp?V
-RTln(Vf/Vi) -1.72 kJ q 1.72 kJ - w
q w ?U ?H 1-2 3.72 0
3.72 6.19 2-3 -6.19 2.47 -3.72 -6.19 3-1
1.72 -1.72 0 0 net -0.75 0.75
0 0
5
2. Find an expression for the change in entropy
when two blocks of the same substance and of
equal mass, one at the temperature Thot and the
other at Tcold are brought into thermal
contact and allowed to reach equilibrium.
Evaluate the change in temperature and Entropy
for two 500-g blocks of copper assuming the molar
heat capacity of Copper to be Cp 24.4 J /(K
mol) and taking Thot 500K and Tcold 250
K. Also, Determine the Entropy change of the
Universe for this process.
6
2. Find an expression for the change in entropy
when two blocks of the same substance and of
equal mass, one at the temperature Thot and the
other at Tcold are brought into thermal
contact and allowed to reach equilibrium.
Evaluate the change in temperature and Entropy
for two 500-g blocks of copper assuming the molar
heat capacity of Copper to be Cp 24.4 J /(K
mol) and taking Thot 500K and Tcold 250
K. Also, Determine the Entropy change of the
Universe for this process.
7
(No Transcript)
8
- A gaseous sample consisting of 1.00 mol molecules
is described by the equation of state - pV RT(1 Bp).
- Initially at 373 K, it undergoes Joule-Thomson
expansion from 100 atm to 1.00 atm. - Given that Cp 5/2R
- µJ-T 0.21 K atm-1
- B -0.525 (K/T) atm-1
- and that these are constant over the temperature
range involved. - Calculate ?T for the gas.
9
3. A gaseous sample consisting of 1.00 mol
molecules is described by the equation of state
pV RT(1 Bp). Initially at 373 K, it
undergoes Joule-Thomson expansion from 100 atm to
1.00 atm. Given that Cp 5/2R µJ-T
0.21 K atm-1 B -0.525 (K/T) atm-1 and that
these are constant over the temperature range
involved, calculate ?T for the gas.