Classification of Matter - PowerPoint PPT Presentation
1 / 8
Title:
Classification of Matter
Description:
... a 10-mL graduated cylinder, measure out 4.0 mL of. 1.0 M HCl solution. Add 5 drops of Universal Indicator. solution to the HCl solution in the graduated cylinder. ... – PowerPoint PPT presentation
Number of Views:23
Avg rating:3.0/5.0
Title: Classification of Matter
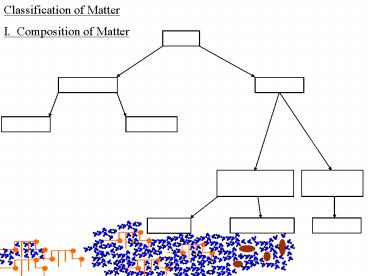
1
Classification of Matter
I. Composition of Matter
2
Classification of Matter
II. Properties of Matter
A. Physical Changes
-a ______ in ____, _____, or _____ of _____
-_______ of _____
-___________ of _______
-________ of ______
-_________ of _____
- still has _____ _________ _________
B. Chemical Changes
-a ______ in ____ ________ in a ________ to a
________ _________
-_______ of _____
-_______ of _______
3
Classification of Matter
II. Properties of Matter
B. Chemical Changes
-________ of ________ _______
1. Energy
-______ is _______ or ________
-____, _____, or ______
2. Chemical
-_________ of ____ ________
-_________ of ________ ___ _______ in _______
-_________ of ________ _____ __________ (______
out of ________)
-_____ _______
-___ ______ (________ or ________ in _______ or
_______), _____ _______of ________
4
Classification of Matter
II. Properties of Matter
B. Chemical Changes
1. Hypothesis
2. Prediction
3. Gather Data
A. Safety
5
Classification of Matter
II. Properties of Matter
B. Chemical Changes
3. Gather Data
B. Procedure
1. Using the top-loading electronic balance,
mass 0.5 g of NaHCO3 into an evaporating
dish.
2. Using a 10-mL graduated cylinder, measure out
4.0 mL of 1.0 M HCl solution. Add 5 drops
of Universal Indicator solution to the
HCl solution in the graduated cylinder.
Add the HCl solution to the contents of the
evaporating dish. Record evidence of
chemical change ____________
______________________________________________
http//www.cartage.org.lb/en/themes/Sciences/Chemi
stry/Analyticalchemistry/LabEquipment/LabEquipment
.htm
6
Classification of Matter
II. Properties of Matter
B. Chemical Changes
3. Gather Data
B. Procedure
3. Repeat Step 2, adding a second measure of HCl
to the contents of the evaporating dish.
Record evidence of chemical
change_________________________________
4. Heat the contents of the evaporating dish to
dryness on the hot plate.
4. Analyze Data
A. In the lab, you massed out 0.5 g of NaHCO3.
Is NaHCO3 a substance or a
mixture?_______________________________
B. Is NaHCO3 an element or compound?_____________
_______
7
Classification of Matter
II. Properties of Matter
B. Chemical Changes
4. Analyze Data
C. In the lab, you added 4.0 mL HCl solution,
which is made by dissolving 36.5 g of HCl
into enough water to make one liter. Is the
HCl solution a substance or a mixture?____________
__
D. Is HCl an element or a compound?______________
5. Draw Conclusions
E. When HCl solution was added to the NaHCO3 the
first time, did a chemical change take
place?______ How did you know?
__________________________________________________
_
8
Classification of Matter
II. Properties of Matter
B. Chemical Changes
5. Draw Conclusions
F. After you added a second 4.0 mL HCl solution
to your evaporating dish and heated the
material in the dish to dryness, a white
crystalline solid was left over in the dish. Was
this white crystalline solid NaHCO3?______
How do you know? ____________________________
_______________________
G. What do you think the white crystalline solid
in the evaporating dish was?_______________
H. What do you think the colorless, odorless,
gas given off when you added the HCl
solution to the NaHCO3 was? ___________