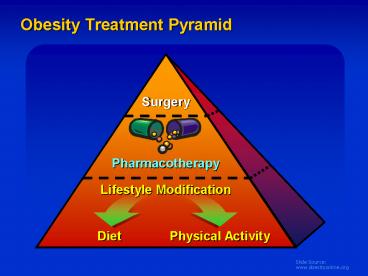
Obesity Treatment Pyramid - PowerPoint PPT Presentation
1 / 47
Title:
Obesity Treatment Pyramid
Description:
The Practical Guide: Identification, Evaluation, and Treatment of Overweight and ... Exenatide (Byetta); incretin mimetic. Enhances insulin secretion ... – PowerPoint PPT presentation
Number of Views:276
Avg rating:5.0/5.0
Title: Obesity Treatment Pyramid
1
Obesity Treatment Pyramid
2
Guide for Selecting Obesity Treatment
BMI Category (kg/m2)
Treatment 25-26.9 27-29.9 30-34.9 35-39.9 gt40
Diet, Exercise, Behavior Tx
Pharmaco-therapy With co-morbidities
Surgery With co-morbidities
The Practical Guide Identification, Evaluation,
and Treatment of Overweight and Obesity in
Adults. October 2000, NIH Pub. No.00-4084
3
Obesity and Dietary Therapy Duct Tape
4
Short-term Obesity Therapy Does Not Result in
Long-term Weight Loss
Diet alone Behavior therapy Combined therapy
Change in Weight (kg)
5-yearFollow-up
1-yearFollow-up
End ofTreatment
Baseline
Wadden et al. Int J Obes 198913 (Suppl 2)39.
5
(No Transcript)
6
Sustained Weight Loss Can Be Achieved with
Behavior Modification Therapy
No Active Treatment
Active Treatment
Women
Weight Loss (kg)
Men
0
2
4
6
8
10-12
Years
Björvell and Rössner. Int J Obes Relat Metab
Disord 199216623.
7
Cardinal Behaviors of Successful Long-term Weight
ManagementNational Weight Control Registry Data
- Self-monitoring
- Diet record food intake daily, limit certain
foods or food quantity - Weight check body weight gt1 x/wk
- Low-calorie, low-fat diet
- Total energy intake 1300-1400 kcal/d
- Energy intake from fat 20-25
- Eat breakfast daily
- Regular physical activity 2500-3000 kcal/wk
(eg, walk 4
miles/d)
Klem et al. Am J Clin Nutr 199766239. McGuire
et al.Int J Obes Relat Metab Disord 199822572.
8
Principles of Pharmacotherapy in the Management
of Obesity
9
Regulation of Food Intake
Brain
Central Signals
Stimulate
Inibit
NPY AGRP galanin
Orexin-A Dynorphin ECS/CB1
a-MSH CRH/UCN GLP-I
CART NE 5-HT
Peripheral signals
Peripheral organs
Glucose CCK, GLP-1,Apo-A-IVVagal
afferents Insulin GhrelinLeptinCortisol
Gastrointestinaltract
FoodIntake
Adiposetissue
Adrenal glands
10
Drugs Approved by FDA for Treating Obesity
Generic Name Trade Names DEA Schedule Approved Use Year Approved
Orlistat Xenical None Long-term 1999
Sibutramine Meridia IV Long-term 1997
Diethylpropion Tenulate IV Short-term 1973
Phentermine Adipex, lonamin IV Short-term 1973
Phendimetrazine Bontril, Prelu-2 III Short-term 1961
Benzphetamine Didrex III Short-term 1960
11
Meta-analysis of RCTs Evaluating Effect of
Orlistat Therapy on Weight Loss at 1-Year
Study or Sub-category WMD (random)95 CI
Hollander 1998
Sjostrom 1998
Davidson 1999
Finer 2000
Heuptman 2000
Lindgarde 2000
Rossner 2000
Bakris 2002
Broom 2002
Kelley 2002
Miles 2002
Total (95 CI)
All subjects had type 2 diabetes WMDweighted
mean difference
-10
-5
0
10
5
FavoursTreatment
FavoursControl
Padwal et al. Int J Obes 2003271437
12
Effect of Long-term Orlistat Therapy on Body
Weight
-4.1 kg
Placebo
Change in Weight (kg)
-6.9 kg
Orlistat
Plt0.001 vs placebo
0
52
104
156
208
Weeks
Torgenson et al. Diabetes Care 200427155
13
Gastrointestinal Side Effects of Orlistat Therapy
Year 1 Year 1 Year 2 Year 2
Placebo Orlistat Placebo Orlistat
Fatty/oily stool 5 31 1 8
Increased defecation 7 20 2 2
Liquid stools 10 13 5 8
Fecal urgency 3 10 2 3
Flatulence 3 7 2 3
Flatus with discharge 0 7 0 1
Fecal incontinence 0 7 0 2
Oily evacuation 1 6 0 5
Low plasma vitamin conc
Vitamin A 0.6 0.3 0.8 0
Vitamin D 0.6 5.1 0.8 3.1
Vitamin E 0.9 4.6 0 1.6
Values are percentage of subjects.
Sjostrom et al. Lancet 1998352167.
14
Meta-analysis of RCTs Evaluating Effect of
Sibutramine Therapy on Weight Loss at 1-Year
Study or Sub-category WMD (random)95 CI
McMahon 2000
Smith 2001
McMahon 2002
Total (95 CI)
- All subjects had hypertension
- WMDweighted mean difference
Padwal et al. Int J Obes 2003271437
15
Effect of Continuous vs Intermittent Subutramine
Therapy on Body Weight
Placebo Intermittent sibutramine Continuous
sibutramine
Body Weight Change (kg)
Run-in period
0
4
8
12
16
20
24
28
32
36
40
44
48
Time (wk)
Sibutramine dose15 mg/d.
Wirth and Krause. JAMA 20012861331.
16
Adverse Effects of Sibutramine Therapy
Subjects () Subjects () Subjects () Subjects ()
Adverse Effect Placebo Sibutramine Sibutramine
Headache 18.6 18.6 30.3
Dry mouth 4.2 4.2 17.2
Constipation 6.0 6.0 11.5
Insomnia 4.5 4.5 10.7
Dizziness 3.4 3.4 7.0
Hypertension 0.9 0.9 2.1
Tachycardia 0.6 0.6 2.6
Palpitation 0.8 0.8 2.0
Meridia Package Insert, 2001.
17
Effect of Continuous and Intermittent Phentermine
Therapy on Body Weight
Continuous Dummy
Weight Loss (lbs)
ContinuousPhentermine
Alternate Phentermine and Dummy QOM
0
8
24
28
36
4
12
16
20
32
Time (weeks)
Munro JF et al. Brit Med J 1352, 1968
18
Regulation of Food Intake
Brain
Central Signals
Stimulate
Inibit
NPY AGRP galanin
Orexin-A Dynorphin ECS/CB1
a-MSH CRH/UCN GLP-I
CART NE 5-HT
Peripheral signals
Peripheral organs
Glucose CCK, GLP-1,Apo-A-IVVagal
afferents Insulin GhrelinLeptinCortisol
Gastrointestinaltract
FoodIntake
Adiposetissue
Adrenal glands
19
Gastrointestinal Peptides Hormones
food intake regulation
Anti-obesity potential
digestion and metabolism
Anti-diabetes potential
Modified from Marx, Science 2003 February 7 299
846-849. (in News)
20
GLP-1
- GLP-1 incretin hormone
- Exenatide (Byetta) incretin mimetic
- Enhances insulin secretion
- Suppresses elevated glucagon secretion
- Reduces food intake and body weight
- Slows gastric emptying
- Increase in beta-cell mass
Toft-Nielsen M, et al. J Clin Endocrinol Metab
2001 863717-3723 Drucker DJ. Mol Endocrinol
2003 17161-171 Nielsen LL, et al. Reg Pept
2004 11777-88
21
Neuroendocrinology of Food Intake
RegulationHindbrain as a Target for Peripheral
Satiety Signals
Hypothalamus
ARC
NTS/AP
Vagus
Spinalnerves
CCK
GI tract
Leptin Insulin
PYY
Ghrelin
Amylin other circulating gut peptides
Modified from Marx, Science 2003 February 7 299
846-849. (in News)
22
Open-Label Extension Combined BYETTA Continued
to Reduce Weight
23
Safety and Tolerability Exenatide Open-Label
Extensions
- Exenatide generally well tolerated
- Adverse events
- Nausea (30-40)
- Diarrhea (7)
- Vomiting (9)
- Feeling jittery (5)
- Dizziness (3)
- Headache (3)
24
AmylinA Neuroendocrine Hormone
Amylin ReceptorIdentified
N
N
Amylin Binding Sites in the Brain
Dorsale Raphe
C
C
RAMP 1 or 3
CTR
Nucleus Accumbens
Area Postrema
Beaumont K, et al. Mol Pharm 1993
44493-497 Adapted from Muff R, et al.
Endocrinology1999 1402924-2927
25
Effects of Pramlintide in Type 2 Diabetes
Pooled 120 µg BID Pramlintide Intent to Treat
Populations
Change in Insulin Use ()
Change in A1C ()
Change in Weight (lb)
Week 4
Week 13
Week 26
Week 4
Week 13
Week 26
Week 4
Week 13
Week 26
2.5
6
0
2.0
5
-0.1
1.5
4
-0.2
1.0
3
0.5
-0.3
2
0
-0.4
-0.5
1
-0.5
-1.0
0
-1.5
-0.6
-1
-2.0
-0.7
-2
-2.5
-3
-0.8
-3.0
Data on file, Amylin Pharmaceuticals, Inc.
26
(No Transcript)
27
Endocannabinoid System (CB-1) as a Potential
Target of Action for Modulation of Energy
Homeostasis and Obesity
27
Energy Balance Feeding Behavior
Gastric emptying GI motility
Limbic forebrain Motivation for palatable food
Ghrelin, PYY
Hepatic Lipogenesis Adipose Tissue Metabolism
Glucose Homeostasis
Lipolysis Lipogenesis
Hepatic glucose output Lipogenesis
Glucose uptake Glucose, lipid oxidation
28
The ECS is Overactivated in
28
- Animal models of genetic obesity
- Animal models of diet-induced obesity
- Human obesity
29
Endocannabinoids Stimulate Food Intake in Mice
29
Anandamide 0.001 mg/kg Vehicle
Plt0.05 Plt0.01 vs vehicle
7 6 5 4 3
Food intake (grams/day)
1 3 5 7
Day
Hao S et al. Eur J Pharmacology. 2000
392147-156.
30
30
The ECS is Upregulated in Human Obesity
Plt0.05 vs lean women
Engeli S, et al. Diabetes 2005 5428382843.
31
31
A Mutation in the Enzyme That Degrades
Endocannabinoids is Associated with Increased BMI
Percent of subjects with FAAH 385 A/A genotype by
BMI category
vs normal BMI
Caucasians
African Americans
16 14 12 10 8 5 4
10 8 6 4 2 0
Plt0.05
Plt0.01
Plt0.05
of subjects with FAAH 385 A/A
18.5-24.9 25.0-29.9 gt30.0
18.5-24.9 25.0-29.9 gt30.0
BMI (kg/m2)
BMI (kg/m2)
Sipe JC et al. Int J Obes Relat Metab
Disord.200529755-759.
32
CB1 Blockade Produces a Dose-Related Reduction in
Food Intake in Mice
32
2.0 1.5 1.0 0.5 0.0
Food intake (g)
0.0 0.3 1.0 3.0 10.0
Rimonabant Dose (mg/kg-1)
Wiley JL et al. Br J Pharmacol.
2005145293-300.
33
33
Supporting Evidence
- Adipose tissue metabolism
- EC stimulation with CB1 agonist increases adipose
tissue LPL expression while CB1 blockade inhibits
this effect - CB1 stimulation reduces while blockade increases
adiponectin synthesis - CB1 blockade reverses the histological changes in
adipose tissue produced by diet-induced obesity - EC stimulation reduces the expression of AMP
kinase in visceral fat
Cota D et al. J Clin Invest. 2003112423.
Matias I, et al. XV ICRS Symposium June 24-27,
2005 Clearwater, Fla. Jbilo O, et al. FASEB J.
2005191567-1569.
34
34
The Peripheral ECS in Adipose Tissue
Adipose tissue of obese mice fed a high fat diet
(HFD) plus rimonabant resembles that of lean mice
fed a standard diet (STD)
Jbilo O, et al. FASEB J. 2005191567-1569.
35
ECS Stimulation, Centrally and Peripherally,
Favors Metabolic Processes that Lead to
35
- Weight Gain
- Lipogenesis
- Insulin Resistance
- Dyslipidemia
- Impaired Glucose Homeostasis
36
RIO Rimonabant In Overweight/ObesityCB-1
Blockade in Human Studies
36
(gt6600 patients enrolled)
1
Pi-Sunyer FX.Obes Res. 200412(suppl)08-OR, A27.
37
RIO-Europe and RIO-Lipids Weight Change at 1
Year
37
Completers ITT (LOCF)
0
-1.5
-2.3
-2
-1.8
Placebo
Rimonabant 20 mg
-4
Weight change (kg)
-3.6
-6
-6.6
-8.6
-6.9
-8
-8.6
-10
0
16
32
- ITT
- LOCF
Weeks
Van Gaal et al. The Lancet 2005 365 1389-97.
Despres J-P, et al. N Engl J Med.
20053532121-2134.
38
RIO-NA Weight Change over 2-Years in
Re-randomized Patients
38
Placebo
ITT (LOCF)
Rimonabant 20 mg/PLB
Rimonabant 20 mg
Weight (kg) Change from Baseline over 2 Years
(Mean /- SEM)
Pi-Sunyer FX et al. JAMA 2006295761-775.
39
RIO-NA HDL-C and TG over 2 Years
39
Patients on same treatment for 2 years
ITT, LOCF
HDL-cholesterol
Triglycerides
30
25
20
Change in Triglycerides ()
Change in HDL-cholesterol ()
15
10
5
-15
0
0
24
48
72
104
Weeks
Weeks
Pi-Sunyer FX et al. JAMA 2006295761-775.
40
RIO-Lipids Percent Change in HDL-C and TG Levels
at 1 Year
40
Rimonabant 20 mg Rimonabant 5 mg Placebo
Completers
10 5 0 -5 -10 -15 -20
30 25 20 15 10 5 0
Plt0.001
22.9
0.4
P0.017
Change in TG ()
Change in HDL-C ()
-3.6
15.6
11.8
Plt0.001
-15.7
0 12 24 36
52
Week
0 12 24 36
52
Week
ITT, LOCF
Placebo 11. 8 R5 mg 14.2 (ns v. placebo)
R20 mg 19.1 (plt 0.001 v. placebo)
Placebo 0.0. R5 mg 1.2 R20 mg -12.6 (p
lt 0.001 v. placebo)
Despres J-P, et al. N Engl J Med.
20053532121-2134.
41
RIO-DIABETESResults Weight Changes
End Point Placebo Rimonabant 5 mg Rimonabant 20 mg P-value PLB vs 20 mg
Weight loss (kg) - 1.40.2 - 2.30.2 - 5.30.3 lt0.001
Decrease in waist circumference (cm) - 1.90.3 - 2.90.3 - 5.20.3 lt0.001
of patients with weight loss 10 2.0 6.2 16.4 lt0.001
of patients with weight loss 5 14.5 21.7 49.4 lt0.001
Scheen A. Late Breaking Clinical Trials. ADA
Scientific Session 2005.
42
42
RIO-NA Overall Safety Year 1
Rimonabant
Rimonabant
Placebo
n 607
20 mg n 1219
5 mg n 1214
44.9
49.0
Overall discontinuations
49.1
85.5
83.4
82.0
Subjects with any adverse event
4.5
3.8
3.5
Subjects with any serious adverse event
12.8
9.4
7.2
Subjects discontinued due to adverse event
Pi-Sunyer FX et al. JAMA 2006295761-775.
43
RIO-NA Adverse Events LeadingTo Drug
Discontinuation in Year 1
43
Placebo Rimonabant Rimonabant
(N607) () 5 mg (N1214) () 20 mg (N1219) ()
Psychiatric disorders 2.3 3.6 6.2
Depressed mood disorders 1.3 2.1 2.2
Anxiety 0.3 0.6 1.0
Irritability 0 0.2 0.5
Insomnia 0.2 lt0.1 0.5
Nervous system disorders 1.0 1.2 2.2
Headache 0.3 0.3 0.5
Dizziness 0.2 0 0.7
Gastrointestinal disorders 0.7 0.7 1.6
Nausea 0.2 0.2 0.9
According to MedDRA, in any rimonabant groups
in main SOCs (gt1 ) and in at least 6 patients
(0.5). One patient may report several events
Pi-Sunyer FX et al. JAMA 2006295761-775.
44
RIO-NA Main Adverse Events Leading to Drug
Discontinuation in Year 2
44
Rimonabant
Placebo
20 mg (N333)N ()
5 mg (N300)N ()
(N298)N ()
7 (2.1)
6 (2.0)
4 (1.3)
Psychiatric disorders
4 (1.2)
4 (1.3)
3 (1.0)
Depressed mood disorders
2 (0.6)
1 (0.3)
0 (0)
Anxiety
Patients receiving the same treatment for 2
years
Pi-Sunyer FX et al. JAMA 2006295761-775.
45
Conclusions
- Obesity is a chronic disease
- Modest weight loss (5 -10 of body weight) can
have considerable medical benefits - Lifestyle change (diet and physical activity) is
the cornerstone of therapy - Pharmacotherapy can be useful in properly
selected patients - Bariatric surgery is the most effective therapy
for severe obesity
46
Obese Patients Have Unrealistic Weight Loss Goals
Outcome Weight (lbs) Reduction
Initial 218 0
Dream 135 38
Happy 150 31
Acceptable 163 25
Disappointed 180 17
Foster et al. J Consult Clin Psychol 19976579.
47
(No Transcript)