Kinetics - PowerPoint PPT Presentation
1 / 18
Title:
Kinetics
Description:
Design and analysis of experiments to test rate equations and derive kinetic parameters ... standard reaction enthalpy S = Standard reaction entropy ... – PowerPoint PPT presentation
Number of Views:28
Avg rating:3.0/5.0
Title: Kinetics
1
Kinetics
2
What is Kinetics ?
- Analysis of reaction mechanisms on the molecular
scale - Derivation of rate expressions
- Design and analysis of experiments to test rate
equations and derive kinetic parameters - Theoretical prediction of rate constants
- How can we improve it?
3
Elementary steps
- Discuss examples of elementary steps!
A reaction is elementary if it cannot be
split up in further steps. An elementary
reaction proceeds exactly as expressed by the
reaction equation. A reaction mechanism is a
sequence of elementary steps.
4
The Rate Equation
5
Free Energy and Entropy
- Equilibrium
- ?G 0
- Free energy minimum
- Entropy maximum
6
Free Energy and Entropy
- Reaction ?G ? 0
- free energy gt minimum
- entropy lt maximum
Thermodynamics
Kinetics
7
Chemical Equilibrium
The Chemical Potential
8
Thermodynamics
- Table 2.2 Thermodynamic Data for Important
Catalytic Reactions - Reaction ?Ho (kJ/mol) ?Go
298(K) - NH3 ? ½ N2 3/2 H2 45.9 16.4
- ½N2 3/2 H2 ? NH3 - 45.9 - 16.4
- N2 3 H2 ? 2 NH3 - 91.9 - 32.8
- 2 NO ? N2 O2 - 182.6 -175.2
- CH4 H2O ? CO 3 H2 205.9 142.0
- CH4 ½ O2 ? CO 2 H2 - 35.9 - 86.8
- CH4 2 O2 ? CO2 2 H2O - 802.6 - 801.0
- CH4 ½ O2 ? CH3OH - 275.6 - 111.77
- CO2 3H2 ? CH3OH H2O - 49.3 3.5
- CO 2H2 ? CH3OH - 90.5 - 25.1
- CO H2O ? CO2 H2 - 41.2 - 28.6
- this per two mole of NH3 or NO. Data taken from
the JANAF Thermodynamic Tables.
9
EX Ammonia Synthesis
- N2 3 H2 2 NH3
- ?G -32.8 kJ/mol
- ?H -91.9 kJ/mol
10
The ammonia reactor
N23 H2 2 NH3
?G -32.8 kJ/mol ?H -91.9 kJ/mol
11
Power Rate Laws
Parametrization of the rate
Reaction Order
Example
12
Equilibrium Constant and Free Energy
?G standard Gibbs energy of reaction ?H -
T?S
Van 't Hoff equation ?H standard
reaction enthalpy ?S Standard reaction entropy
13
Temperature dependence of the rate constant
- Eact / RT
- Arrhenius equation k v e
Eact activation energy v preexponential
factor
E
Eact
reactants
products
reaction parameter
14
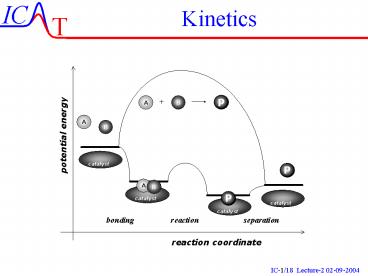
Potential Energy Representationdissociation -
association
15
Temperature Dependence of the Rate
ln k ln v Eact / RT
ln k
1000 / T
16
Apparent Activation Energy
Example
17
Heterogeneous Catalysis
Adsorption
Reaction
Desorption
18
Basic surface interactions
- Reactions take place on the metal surface