Electrophilic Aromatic Substitution - PowerPoint PPT Presentation
1 / 30
Title:
Electrophilic Aromatic Substitution
Description:
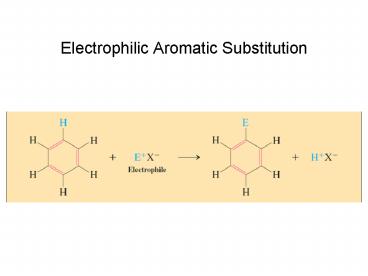
Electrophilic Aromatic Substitution. The most important reaction of benzene. Rate determining step is addition by the electrophile ... – PowerPoint PPT presentation
Number of Views:483
Avg rating:3.0/5.0
Title: Electrophilic Aromatic Substitution
1
Electrophilic Aromatic Substitution
2
EAS Mechanism
3
(No Transcript)
4
Bromination Requires a Catalyst
5
Deprotonation gives Aromatic Structure
6
Phenyl-H 112 kcal mol-1 Br-Br 46 kcal
mol-1 Phenyl-Br -81 kcal mol-1 H-Br -87.5
kcal mol-1 ---------------------------------------
------------------ Reaction -10.5
kcal mol-1
7
Nitration and Sulfonation
8
Nitric Acid activated by Sulfuric Acid
9
Electrophilic Addition of Nitro Group
10
Nitration and Sulfonation
11
Sulfonation is Reversible
12
Sulfonate Applications
13
Sulfonate Applications
14
Sulfa Drugs
15
Friedel-Crafts Alkylation
16
Friedel-Crafts Mechanism
17
Intramolecular Friedel-Crafts
18
Other Carbocation Precursors Can Be Used
19
Friedel-Crafts Alkylation has Limitations
20
Rearrangement Can Also Occur
21
End Lecture 17
22
Friedel-Crafts Acylation
23
Reaction Gives Ketone as Product
24
Acylium Ion Formation
25
Electrophilic Acylation
26
Aqueous workup is required
27
Ketone Reduction
28
Summary
- Electrophilic Aromatic Substitution
- The most important reaction of benzene
- Rate determining step is addition by the
electrophile - Exothermic substitution preferred over
endothermic addition - Leads to halo- and nitrobenzenes, benzenesulfonic
acids, alkylated and alkanoylated derivatives
29
Summary
Sulfonation of benzene is reversible by heating
with dilute aqueous acid Benzenesulfonic Acids
Precursors of benzenesulfonyl chlorides. These
chlorides React with alcohols to form sulfonic
esters containing useful leaving groups React
with amines to give sulfonamides (some of which
are medically important) Friedel-Crafts
Alkylations Activate the aromatic ring to
further electrophilic substitution which leads to
product mixtures
30
(No Transcript)