Estimate the area of the yellow circles - PowerPoint PPT Presentation
1 / 13
Title:
Estimate the area of the yellow circles
Description:
John Dalton. Experimented with reacting gases ... Dalton knew atoms existed and how they combined to make compounds, but he had no ... – PowerPoint PPT presentation
Number of Views:91
Avg rating:3.0/5.0
Title: Estimate the area of the yellow circles
1
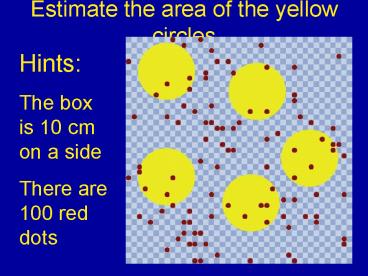
Estimate the area of the yellow circles
Hints The box is 10 cm on a side There are 100
red dots
2
Use the dots to estimate the size of the yellow
circles
- Notice, the dots are evenly distributed in the
area - So you can expect the proportion of dots on
yellow circles to be the same as the proportion
of area of yellow circles - dots in yellow / total dots EQUALS
total area of yellow / total area - 23 dots / 100 dots area of yellow / 100 cm2
- Area of yellow dots 23 cm2
- So each dot is about 5 cm2
3
Thats how the radius of an atomic nucleus was
first measured!
- But were getting ahead of ourselves
- Lets start with a few basic ideas
4
Democritus of Abderra
- Lived in Greece, from about 460 B.C. to 370 B.C.
- Along with Leucippus, was first to suggest that
all matter is made of microscopic atoms - Reached that conclusion using ONLY logic never
conducted any experiments to check the idea - (also first to realize the Milky Way was made of
millions of separate stars)
5
John Dalton
- Experimented with reacting gases
- Example Observed that water could be broken
down into hydrogen and oxygen but their masses
were not equal. 18 grams of water would give 2
grams of hydrogen and 16 grams of oxygen. And
NEVER any other gas! - This, and many other observations with other
gases, led to
6
Daltons Atomic theory
- Elements are made of very tiny particles called
atoms - All atoms of a given element are identical
- Atoms of different elements have different
properties, including mass and chemical
reactivity - Atoms arent changed by chemical reactions they
are merely rearranged into new combinations - Compounds are formed when atoms of different
elements combine - Compounds are defined by the number and type of
the constituent atoms
7
This theory could be falsified by
- Some examples
- Breaking water down into two elements other than
hydrogen and oxygen - Finding atoms of an element that have some
dissimilar properties - Observing a chemical reaction that changes the
atoms involved
8
What about the structure of the atoms themselves?
- Dalton knew atoms existed and how they combined
to make compounds, but he had no evidence
concerning their structure
- So he picked the simplest possible structure
tiny, hard spheres
9
J.J. Thomson (1897)
- Discovered the electron
- Conducted experiments in which a gas produced
electrons - Concluded that atoms are positively charged
spheres that contain removable electrons
10
Ernest Rutherford (1911)
- Bounced alpha particles off of atomic nucleus
- Realized that positive part of atom must be tiny,
less than 1/10,000th of the volume! - Did not have information about electron location
- Discovered the proton in 1918
11
Niels Bohr (1913)
- Used observations that each element will emit
particular frequencies of light to suggest that
electrons only exist in precisely defined orbits
12
Erwin Schrodinger (1926)
- Louis de Broglie showed that a moving particle
could be treated, mathematically, as a wave - Schrodinger used that result to work with
electrons in atoms as probability waves. - This led to much more precise predictions of the
light emitted by atoms
13
James Chadwick (1932)
- Identified where the extra mass in atoms came
from. - There is another particle in the nucleus - the
neutron. It has the same mass as a proton, but
is electrically neutral.