Molecular Adsorbate on Metal Surfaces - PowerPoint PPT Presentation
1 / 16
Title:
Molecular Adsorbate on Metal Surfaces
Description:
that can be detected using spectroscopies ... that Ead for CO on Pd(111) has a precipitous drop just at =0.5 due to a c(4x2) reconstruction. ... – PowerPoint PPT presentation
Number of Views:80
Avg rating:3.0/5.0
Title: Molecular Adsorbate on Metal Surfaces
1
Molecular Adsorbate on Metal Surfaces
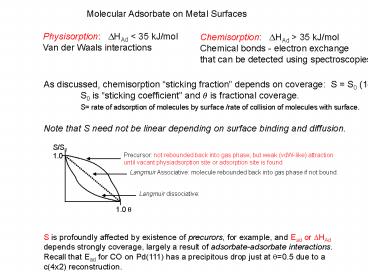
Physisorption ?HAd lt 35 kJ/mol Van der Waals
interactions
Chemisorption ?HAd gt 35 kJ/mol Chemical bonds
- electron exchangethat can be detected using
spectroscopies
As discussed, chemisorption sticking fraction
depends on coverage S S0 (1-?) S0 is
sticking coefficient and ? is fractional
coverage. S rate of adsorption of molecules by
surface /rate of collision of molecules with
surface. Note that S need not be linear
depending on surface binding and diffusion.
S is profoundly affected by existence of
precurors, for example, and Ead or ?HAd depends
strongly coverage, largely a result of
adsorbate-adsorbate interactions. Recall that
Ead for CO on Pd(111) has a precipitous drop just
at ?0.5 due to a c(4x2) reconstruction.
2
Example of Dissociation with Precursor Diatomic
O2
- A adsorption, Ddisassociative adsorption.
- (i) interaction of O with surface
- (ii) interaction of O2 with surface
- non-activated process, i.e. no barrier,
- so ?HAd,O2 ?HD,O2
- Ediss activation barrier to dissociation
- (via surface adsorption), which is much less than
the gas phase dissasociate energy D(OO). - Hence the surface facilitates dissociation with
respect to gas phase. - Activated adsorption corresponds to crossing pt.
- Non-activated adsorption occurs when crossing
pt. is below zero of energy.
Diagrams not from Hoffman are from Attard and
Barnes, Surfaces (Oxford Chemistry Primer, 2001)
3
Molecular Orientation, Vibration, and Rotation
Diatom
- What affects rate of dissociation?
- Orientation arising from difference in extent of
overalap between antibonding orbital on molecule
and filled electronic state in solid. - Vibrational excitation
- Bond length in diatomic molecule is longer on
average in excited state. - Rotational excitation
- Leads to increas in bond length, but poor orbital
overlap between molecule and surface favors one
state over another, e.g. - helicopter favored over cartwheel, for
sticking probability.
4
Gaseous Phase Dissociation H2
Two effects occur Broadening of energy range of
MO of H2, relative to free molecule, due to
mixing of the w.f. between the molecule and
metal. Two new sets of molecular orbitals form
(? bonding and ? antibonding) between the
adsorbed molecule and the metal. With e- transfer
from metal to ? of H2 until energy of HOMO Ef
of metal.
Depending on the extent of e- transfer from metal
to dihydrogen (essentially now in a precursor
state), the H-H bond will weaken until the
bonding component of bond energy is outweighed by
the antibonding component and dissociation occurs.
5
Potential Energy and Dissociation of H2
The extent of e- transfer depends on the relative
positions of Ef and the vacant ? orbital in the
precursor. Metals that readily transfer charge
to the ? orbital will affect dissociation,
whereas those that do not (e.g., Cu) will exhibit
low values of S0.
- Figure diatomic molecule approaches surface
(gas ? precursor ? adsorption) - and ? are seen to split as a function of R.
- What was ? on molecule eventually falls below Ef
and is partially filled with e- from metal. - The filling of ? orbitals by e- from metal is
refereed to as back-donation. - When diatom dissociates, forming chemisorbed
atoms, the orbitals collapse to M-H ? and M-H ?.
- PES (photo-electron-spectroscopy) can be used to
monitor t-dependent changes in orbitals.
6
Hoffmanns diagram for rigid H2 at adsorption
7
Orbital interactions that (de)stabilize adsorbate
Perturbation theory level splitting ?E
Hij2/(Ei0 - Ej0)
Filled levels do not hybridize and stabilize.
??
??
??
??
No effect
??
??
??
??
attraction
8
CO on Ni(001) again
5? is interaction with d? (z2 band) would be
repulsive, mainly due 4-electron two-orbital
interactions, were it not for pushing some
antibonding above Ef. RESULTS loss of z2 DOS and
concomitant bonding!
O C
carbonyl lone pair 5?
d-hybrid spdz2
dxz, dxz (the d?) CO ?-states
9
Chemisorption is compromise
1 and 2 help bind molecule But weaken bonding in
molecule and In surface atoms. 3 repulsive and 4
no effect
Stabilizes by dumping electrons
10
CH3 interactions with a surface
Atop sites
Bridge sites
smallest
11
Examples of Work Fct. ? changes
12
UPS experiments Low-E photons exhibit much
narrower energy spread than XPS, which is at 1
eV. Source energies HelI (21.22 eV) and NeI
(16.85 eV) observe 10eV range of binding.
- Eb h? - Ekin - ?
- ? work fct.
- h? photon energy
- Ekin K.E. observed
13
Example Spectra from UPS
14
UPS for molecular orientation
Spectra does not vary much as angle is
changed. Implies vertical orientation. Can you
think of another spectral method that could give
orientational info?
15
Reflection-adsorption infrared spectroscopy RAIRS
IR spectra observe IR modes What are dipole
moments?
C?O
C?O
images charges in metal
dipoles cancel dipoles enhance
Make E field perpendicular or parallel to
surface. Perpendicular enhance dipoles if
vertical.
16
High-resolution electron energy loss
spectroscopy HREELS
Vibrational modes Hollow and atop x and y
frustrated translations are degenerate. Bridge x
and y are different.
For dissociative adsorption of H on W(001),
HREELS observe 3 line spectra indicating bridge
sites.