Precipitation Titrations - PowerPoint PPT Presentation
1 / 8
Title:
Precipitation Titrations
Description:
Cl-, Br-, I-, CN-, SCN-, CNO-, IO3-, S2-, AsO43-, CrO42 ... The precipitate must adsorb its own ions. The pH must not be too acidic - must produce Fl- ions ... – PowerPoint PPT presentation
Number of Views:2007
Avg rating:3.0/5.0
Title: Precipitation Titrations
1
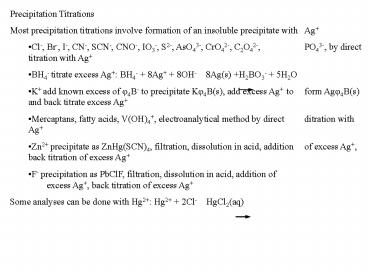
- Precipitation Titrations
- Most precipitation titrations involve formation
of an insoluble precipitate with Ag - Cl-, Br-, I-, CN-, SCN-, CNO-, IO3-, S2-, AsO43-,
CrO42-, C2O42-, PO43-, by direct titration with
Ag - BH4- titrate excess Ag BH4- 8Ag 8OH-
8Ag(s) H2BO3- 5H2O - ? add known excess of f4?- to precipitate
Kf4?(s), add excess Ag to form Agf4?(s) and
back titrate excess Ag - Mercaptans, fatty acids, V(OH)4,
electroanalytical method by direct ditration
with Ag - Zn2 precipitate as ZnHg(SCN)4, filtration,
dissolution in acid, addition of excess Ag,
back titration of excess Ag - F- precipitation as PbClF, filtration,
dissolution in acid, addition of excess Ag,
back titration of excess Ag - Some analyses can be done with Hg2 Hg2 2Cl-
HgCl2(aq)
2
- Precipitation Titrations
- For the reaction between Ag and A- AgA Ag
A- KspAgA- - Classify regions of titration curve when Ag is
titrant - Initial point VAg 0 mL, no Ag, A- CA-
- Add Ag, prior to equivalence point common ion
problem with A- in excess - Equivalence point solubility of salt in water
- Beyond equivalence point common ion problem with
Ag in excess - Example Titrate 50.00 mL of 0.1000 M Cl- with
0.1000 M Ag - Initial point 0.00 mL added Ag solution
- Cl- CCl- 0.1000 M pCl 1.00
- Ag 0 pAg ?
- Add 1.00 mL Ag solution
- AgCl Ag Cl- Ksp AgCl- 1.82
x 10-10
3
- Precipitation Titrations
- Example Titrate 50.00 mL of 0.1000 M Cl- with
0.1000 M Ag - Add 49.90 mL 0.1000 M Ag
- Equivalence point Ag Cl-
- Add 50.01 mL 0.1000 M Ag or 0.10 mL beyond
equivalence point - AgCl Ag Cl-
4
- Precipitation Titrations
- Effect of Concentration of Reagents See Figure
13-1, FAC7, p. 267 - Effect of size of Ksp see Figure 13-2, FAC7, p.
268 - Indicators
- For AgA Ag A-
- InA In A-
- color 1 color 2
- see color 1 if
- see color 2 if
5
- Precipitation Titrations
- Mohr Method makes use of a second, colored
precipitate for indicator action - CrO42- is used as indicator Ag2CrO4 is brick red
- Ag2CrO4 is more soluble than AgCl
- As Ag is added to a solution of Cl- and CrO42-,
AgCl forms first until Cl- is low enough for
CrO42- to begin reacting with Ag - If Ag2CrO4 begins precipitating when Cl- is at
the equivalence point concentration - AgCl Ag Cl-
- Ag2CrO4 2Ag CrO42-
- 6 x 10-3 CrO42- is too colored to see the brick
red Ag2CrO4 - Thus 2 x 10-3 M CrO42- is used the titration
will over consume Ag - Correct the over consumption with an indicator
blank - The analytical Solution must be buffered
- 2CrO42- 2H 2HCrO42- Cr2O72-
H2O - 2Ag 2OH- 2AgOH Ag2O
H2O
Use borax
6
- Precipitation Titrations
- Volhard Method form a colored coordination
compound with Fe3 - Ag is titrated with SCN-
- AgSCN Ag SCN-
- Fe3 SCN- FeSCN2
- blood red
- If Fe3 ? 0.01 M, negligible error is
introduced Kf(FeSCN2) 103 - Analytical solution must be acidic to prevent
formation of Fe(OH)3 - Usual application - indirect determination of
hilides by adding a known excess of Ag and
back titrating the excess with a standard
solution of SCN- - AgCl is more soluble than AgSCN AgCl SCN-
AgSCN Cl- - This results in an fading endpoint and over
consumption of SCN- - Isolate the AgCl from the solution either by
filtration or coating AgCl(s) with nitrobenzene
7
Precipitation Titrations Fajans Method makes use
of an adsorption indicator, such as
dichlorofluorescein or tetrabromofluorescein
Fl- not high enough to precipitate AgFl In the
titration of Cl- with Ag, prior to the
equivalence point, Cl- ions are in the primary
adsorption layer, and Fl- is found in bulk
solution colored yellow-green Beyond the
equivalence point, Fl- is in the counter ion
layer and shows a pink color
- HFl H Fl-
- yellow-green
- Fl- Ag AgFl(s)
- pink
8
- Precipitation Titrations
- Fajans Method makes use of an adsorption
indicator - Additional requirements
- Make sure colloidal AgCl is formed - add dextrin
- The precipitate must adsorb its own ions
- The pH must not be too acidic - must produce Fl-
ions - The pH must not be to basic - avoid precipitation
of AgOH and formation of Ag2O