Potentiometric Precipitation Titration Example - PowerPoint PPT Presentation
1 / 18
Title:
Potentiometric Precipitation Titration Example
Description:
Potentiometric Precipitation Titration Example ... This creates anion vacancies in the crystal and analyte, such as F- can diffuse ... – PowerPoint PPT presentation
Number of Views:1227
Avg rating:3.0/5.0
Title: Potentiometric Precipitation Titration Example
1
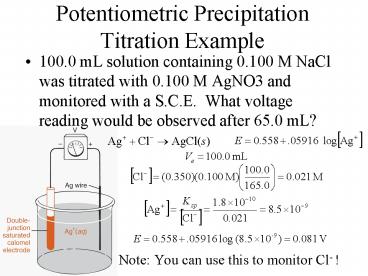
Potentiometric Precipitation Titration Example
- 100.0 mL solution containing 0.100 M NaCl was
titrated with 0.100 M AgNO3 and monitored with a
S.C.E. What voltage reading would be observed
after 65.0 mL? - Note You can use this to monitor Cl- !
2
Error due to Junction Potential
A voltage difference develops when dissimilar
electrolyte solutions are in contact. This
happens at the salt bridge/solution interface.
The junction potential is a major (fundamental)
source of error in a potential measurement,
because the voltage contribution due to the
junction potential is not known. The junction
potential develops because different ions have
different mobilities in water. This creates
regions of slight excess either positive or
negative charge.
3
Ion Mobilities and LJ Potentials
K and Cl- have similar mobilities in water which
is why KCl is so commonly used as an electrolyte
in the salt bridge
4
Ion-Selective Electrodes
- ISEs respond to one ion
- They do not involve redox reactions
- Utilize a membrane that binds only one ion
- The electric potential across the membrane
depends on the concentration of analyte - The difference in voltage is measured by two
reference electrodes
Binding Agent
Analyte
5
Potential Difference for an ISE
- If we describe the electric potential difference
across the boundary between the membrane and
analyte solution - The potential difference between the analyte
solution and the solution inside the ISE is - But almost all of these are constants, so we can
simplify the equation significantly
6
Glass Electrode (pH Meter)
- The pH meter is the most common ISE
The pH meter uses two Ag/AgCl reference
electrodes to measure the potential across a
glass membrane that allows only H to pass
through. H can replace cations bound to
oxygen in glass, called an ion-exchange
equilibrium.
7
The Glass Membrane in a pH Meter
H is the only ion that binds significantly to
the outer (hydrated) layer of glass
? is the electromotive efficiency, which is close
to 1.0 and is measured during calibration.
8
Calibration of a pH Meter
A pH meter must be calibrated with several
buffers close to the pH of the unknown. There
are obviously many buffers that can be used for
this (anything with a Ka) It is also
important that the pH meter be kept in solution
when not in use. If not, the hydrated gel layers
of the glass will dry out and the electrode will
have to be reconditioned for several hours before
use.
9
Sources of Error with a pH Meter
- Calibration standards (0.01 pH)
- Junction potential (0.01 pH)
- Junction potential drift (recalibrate 2 hrs)
- Sodium error (when H is low and Na is high)
- Acid error
- w/ strong acid, the glass surface can saturate
- Equilibration time (Electrode requires 30s)
- Hydration of glass (If the electrode is dry)
- Temperature
- Must be calibrated at same T as measurements
10
Other Ion Selective Electrodes
ISEs are used for many analytes. There are
a number of different types of ISEs 1) Glass
membrane electrodes (H) 2) Solid state
electrodes 3) Liquid-based electrodes 4)
Compound electrodes
11
Selectivity Coefficient
- The selectivity coefficient describes the
relative response of the electrode to different
species - What this means is that for the electrode
measuring A, the smaller k is, the less
interference there is due to ion X
12
Solid-State ISEs
Solid-state ISEs are based on an inorganic
crystal. e.g. LaF3 doped with EuF2. This
creates anion vacancies in the crystal and
analyte, such as F- can diffuse into the crystal
and jump from one side to the other across the
vacancies.
13
Solid-State Electrode Analytes
14
Liquid-Based ISEs
Liquid-based ISEs use a mobile carrier to
transport an ion across a membrane. e.g. for the
Ca2 ISE on the left, the membrane contains a
hydrophobic species that is a chelator of
Ca2. Equilibration of Ca2 with the chelator
establishes a voltage at the membrane-solution
boudary related to Ca2
15
Liquid-Based ISE Analytes
16
Compound ISEs
Compound ISEs contain a normal electrode with a
membrane to isolate the analyte. These
electrodes can be used to measure many different
gases. The electrode at left is for CO2
17
Solid State Sensors
Solid state sensors, especially field effect
transistors (FETs), are analytical designed for
detection of analytes in a solid phase rather
than liquid/aqueous. FETs use
microchips but operate on principles similar to
the pH electrode. FETs are frequently
used for detection of analytes in soil samples
(nitrate, phosphate)
18
Specifications for Electrochemical Techniques
- Advantages
- Linear response to analyte over wide dynamic
range - Nondestructive
- Short response times
- Unaffected by color/turbidity (limited matrix
effects) - Cheap
- Disadvantages
- Sensitivity (High detection limits)
- Not universal