Ozone - PowerPoint PPT Presentation
1 / 23
Title:
Ozone
Description:
to ocean and atmosphere. Nonvolatiles stayed in crust, ... adsorb to mineral particles. Carboxylates in humus provide additional. cation exchange capacity ... – PowerPoint PPT presentation
Number of Views:70
Avg rating:3.0/5.0
Title: Ozone
1
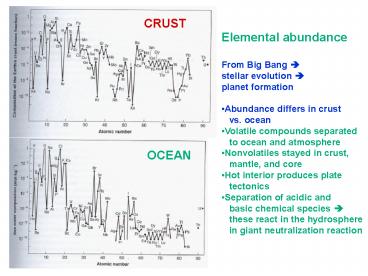
CRUST
- Elemental abundance
- From Big Bang ?
- stellar evolution ?
- planet formation
- Abundance differs in crust
- vs. ocean
- Volatile compounds separated
- to ocean and atmosphere
- Nonvolatiles stayed in crust,
- mantle, and core
- Hot interior produces plate
- tectonics
- Separation of acidic and
- basic chemical species ?
- these react in the hydrosphere
- in giant neutralization reaction
OCEAN
2
CO2 links the atmospheric carbon cycle and
photosynthesis, with the carbonate reactions in
the lithosphere (inorganic carbon
cycle) Rock weathering CO2 H2O CaCO3 ?
Ca2 2 HCO3- This is one aspect of the
inorganic carbon cycle
3
- Chemical evolution of Earth
- acid-base separation
- Atmosphere (volatile) acids CO2, SO2
- Lithosphere bases
- basic oxides of Na, K, Mg, Ca
- in a silicate framework
- calcium carbonate (limestone)
Silica network solids with tetrahedral
Si (SiO2)
Silicate minerals other metals take the place
of some Si atoms, so metal oxides are
incorporated Weathering of silicate minerals then
produces secondary minerals
4
(SiO2)n is electrically neutral, Si 4 Si can
be replaced by Al3 in many minerals,
eg NaAlSi3O8 (feldspar) Al2Si2O5(OH)4
(kaolinite)
Weathering of feldspar to produce kaolinite 2
NaAlSi3O8 2 CO2 11 H2O ? 2 Na 2 HCO3- 4
Si(OH)4 Al2Si2O5(OH)4
5
(No Transcript)
6
- CO2 links the atmospheric
- carbon cycle and
- photosynthesis, with the
- carbonate reactions in the
- lithosphere (inorganic
- carbon cycle)
- Organic C cycle
- Photosynthesis/respiration
- Combustion
- Burial of reduced carbon
- and reoxidation thru
- geological uplift
7
- CO2 links the atmospheric
- carbon cycle and
- photosynthesis, with the
- carbonate reactions in the
- lithosphere (inorganic
- carbon cycle)
- Inorganic C cycle
- Rock weathering
- Precipitation of CaCO3 in
- the oceans
- Conversion of carbonate
- to calcium silicates CO2
Geologic phases of both cycles operate
over millions of years
8
Inorganic carbon cycle Dissolution of limestone
(weathering) removes CO2 from atmosphere CO2
H2O CaCO3 Ca2 2HCO3- Precipitation of
carbonate in oceans results in CO2
outgassing Ca2 CO32- CaCO3(s) 2 HCO3-
CO32- H2CO3 H2CO3 CO2 H2O Weathering of
terrestrial carbonates consumes the same amount
of CO2 as is released when carbonates
precipitate in the ocean.
9
CO2 sequestration Dissolution of limestone
(weathering) removes CO2 from atmosphere CO2
H2O CaCO3 Ca2 2HCO3- Reacting a CO2
emissions stream with basic minerals amounts
to speeding up the weathering process Reactions
with limestone (CaCO3) would require liquid
disposal (deep ocean) Another possibility is to
react with magnesium silicates MgSiO3 H2CO3
H2O ? Si(OH)4 MgCO3 Magnesium carbonate
bricks can be buried, but the mineral reaction is
slow
10
T -63C, P 0.01
T 500C, P 100
T 15C, P 1
Development of photosynthesis explains the
abundance of oxygen on Earth Venus too hot to
allow liquid water ? lost to space, and CO2 could
not be trapped as carbonates in the
lithosphere Mars too small and cold, lost
internal tectonic activity weathering lowered
CO2 concentration w/o replenishment ? greenhouse
effect gone, and all water freezes Earth
liquid water on surface maintains tectonic
activity
11
(No Transcript)
12
Carbonate chemistry in natural waters
1.
2.
4.
3.
inorganic portion of global C cycle
- There are many relevant equilibria in the carbon
system - CO2(g) H2O(aq) H2CO3 (aq) gas in air with
the aqueous acid - H2CO3 H HCO3- acid dissociation to
bicarbonate - CaCO3(s) Ca2 CO32- dissolution of carbonate
from rock - CO32- H2O HCO3- OH- base dissociation
to bicarbonate
13
Carbonate chemistry in natural waters
Calcium carbonate equilibrium Ksp
Ca2CO32- 4.6 x 10-9 Dissolved carbonate
acts as a base in water according to
reaction 4. Kb HCO3-OH- / CO32-
1.
2.
4.
3.
Unless the solution is very alkaline, the
equilibrium for reaction 4. favors production
of HCO3-. 3. CaCO3(s) Ca2 CO32- 4.
CO32- H2O HCO3- OH-
- CaCO3(s) H2O Ca2 HCO3- OH-
- Ksp Ca2HCO32-OH-
14
Carbonate chemistry in natural waters
1.
Calcium carbonate equilibrium Ksp
Ca2CO32- 4.6 x 10-9 Ksp is an equilibrium
constant that depends on temperature
2.
4.
3.
Solubility of CaCO3 in water Ca2 CO32- S
6.8 x 10-5 M S is affected by the other
equilibria. The CO32- ion is a base, and this
will increase CaCO3 solubility (reaction
4.) Atmospheric equilibria increases solubility
further, because the H produced in reaction 2,
combines with the OH- from reaction 4.
15
Some marine organisms with CaCO3 shells
Increased CO2 in ocean leads to higher H
dissolution of CaCO3 Saturation horizon shifts
up deeper colder, more acidic waters are more
undersaturated ? shells more prone to dissolve
Approximate overall pH decrease to data 0.1
unit Projected 0.3 unit decrease by 2100 in
shallow waters
16
Decreased pH increases CaCO3 solubility Colder
temperature increases CaCO3 solubility Coral
bleaching occurs at warmer temperature
17
aragonite shell magnesium calcite shell
- Aragonite and calcite different crystal forms of
CaCO3 - Some calcite minerals substitute Mg2 for some
Ca2 - Aragonite and magnesium calcite are more soluble
than calcite, - and are more susceptible to CO2-generated
acidification
18
Aragonite oversaturation and undersaturation
Pteropod (aragonite) shell in undersaturated
waters
normal shell
19
(No Transcript)
20
(SiO2)n is electrically neutral, Si 4 Si can
be replaced by Al3 in many minerals,
eg NaAlSi3O8 (feldspar) Al2Si2O5(OH)4
(kaolinite) To maintain electrical neutrality,
Na, K, Mg2 or other metal may attach
to a particle. Leads to ion exchange effects
(cation exchange capacity CEC) Protons also
exchange ? silicate clays can help
neutralize acidic soil This also underlies
leaching of metals due to soil acidity
Cation exchange depends on preference of
cation for binding to anionic sites on clay
vs. solvation in water
21
SOIL TYPES DEFINED BY PARTICLE SIZES
Soils mineral, organic matter, water and air
Organic matter humus partly decomposed
plants including protein and lignin Some OH in
lignin oxidize to COOH ? adsorb to mineral
particles Carboxylates in humus provide
additional cation exchange capacity
LIGNIN
22
Hardness of water
Limestone neutralization AND cation exchange on
clay BOTH leach Ca2 and Mg2 ? hardness of the
water increases
Ca2 STP ?
- Hardness index Ca2 Mg2
- Measured by titration with EDTA
- Hard water gt 150 mg/L
- Hard water forms insoluble salts with
- anions in soaps
- Detergent builders are added
- chelating agents such as
- sodium tripolyphosphate (STP)
- Then STP ? Na3PO4 ? eutrophication
- in natural waters
23
Heavy metal contamination in soils and sediments
Havy metals magnify damage from
acidification Typical contaminants Cd, Cu, Ni,
Pb, Zn Leaching occurs, as for Ca2, Al3
- Sediments river, lake and ocean bottoms
- Both soils and sediments are sinks
- for accumulation of pollutants
- sulfides control availability of metals
- Hg2 S2- ? HgS (s) Ksp 3 x 10-53
- (metals available when in excess of
- S2- concentrations)
- sediment excavation and analysis can
- yield an historical record
- Buffering capacity of soils, eg carbonate,
- can disguise acidification
Halifax harbor sediments, Nova Scotia