Composition of Earth - PowerPoint PPT Presentation
1 / 10
Title:
Composition of Earth
Description:
Rocks are not elemental, they are heterogeneous mixtures of minerals. Minerals are crystalline inorganic substances that have specific chemical composition. ... – PowerPoint PPT presentation
Number of Views:254
Avg rating:3.0/5.0
Title: Composition of Earth
1
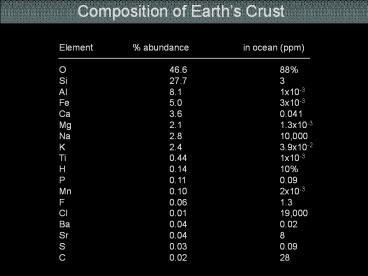
Composition of Earths Crust
Element abundance in ocean (ppm) O 46.6 8
8 Si 27.7 3 Al 8.1 1x10-3Fe 5.0 3x10
-3 Ca 3.6 0.041 Mg 2.1 1.3x10-3 Na 2.8
10,000 K 2.4 3.9x10-2 Ti 0.44 1x10-3 H
0.14 10 P 0.11 0.09 Mn 0.10 2x10-3 F
0.06 1.3 Cl 0.01 19,000 Ba 0.04 0.02 Sr
0.04 8 S 0.03 0.09 C 0.02 28
2
Rocks and Soil
Rocks are not elemental, they are heterogeneous
mixtures of minerals Minerals are crystalline
inorganic substances that have specific chemical
composition. EX CaCO3, Fe2O3, Bi2S3 Most
common minerals (examples) silicates quartz
SiO2, olivine (Mg/Fe)2SiO4, feldspars
Na/KAlSi3O8 oxides corundum Al2O3, hematite
Fe2O3 carbonates calcite CaCO3, dolomite
MgCO3 sulfides pyrite FeS2, galena
PbS sulfates gypsum MgSO4 halides halite
NaCl, fluorite CaF2 native elements sulfur,
platinum, copper, silver, gold Soil is composed
of inorganic and organic materials inorganic
components are clays and weathered rocks
organic components are from plants, fungi,
microbes, animal waste
3
Silicates
Silicates are the most abundant mineral class on
Earth SiO2 silica, quartz, sand,
amythests completely insoluble in water does not
affect the pH of water anionic silicates large
number of minerals fall into this category low
solubility in water increases pH of water
(basic) reacts with acids SiO3- 2H H2O ?
Si(OH)4 silicic acid note silicic acid
condenses readily to form SiO2 and
H2O weathering of silicate minerals liberates
ions to water (Ca2, Fe2, etc.) weathering of
silicate minerals by H2CO3 results in formation
of carbonates CaSiO3 H2CO3 H2O ? SiO2
2H2O CaCO3
4
Aluminates
Al2O3 alumina, corundum, bauxite insoluble in
water does not affect the pH of water anionic
aluminates AlO2- , spinel, beryls, etc. low
solubility in water increase pH of water
(basic) reacts with acids AlO2- H H2O ?
Al(OH)3 aluminum hydroxide note aluminum
hydroxide condenses over time to form Al2O3 and
H2O or AlO(OH) beohmite, diaspore weathering
of aluminate minerals liberates ions to water
(Ca2, Fe2, etc.) weathering of aluminate
minerals by H2CO3 results in formation of
carbonates CaAl2O4 H2CO3 H2O ? Al2O3
2H2O CaCO3
5
Aluminosilicates
Imagine an SiO2 repeating network substitute an
Al for an Si atom Al3 has one less positive
charge than Si4, so there needs to be an extra
cation to maintain electroneutrality. Ex SiO2
Si10O20 substitute one Al3 AlSi9O20-
balance the charge, say with a sodium
cation NaAlSi9O20 -- this is an
aluminosilicate Aluminosilicates are the
component of clays Aluminosilicates have ion
exchange properties NaAlSi9O20 HCl ? NaCl
HAlSi9O20 2NaAlSi9O20 CaCl2 ? 2NaCl
Ca(AlSi9O20)2 (in this example, the acidic
proton was captured by the clay) Clays neutralize
acidic rainwater
6
Weathering of Clays
a bit of extra information for anyone interested
in geology Let us suppose that clays are being
weathered by rain, which is slightly acidic due
to atmospheric CO2 3KAlSi3O8 (feldspar)
2H2CO3 H2O ? KAl3Si3O10(OH)2 (mica) 6H4SiO4
2K 2HCO3- KAl3Si3O10(OH)2 (mica) H2CO3
3/2 H2O ? 3/2Al2Si2O5(OH)4 (kaolinite) K
HCO3- 3/2Al2Si2O5(OH)4 (kaolinite) 15/2 H2O ?
3 Al(OH)3 (gibbsite) 3H4SiO4 note H4SiO4 is
Si(OH)4 Si(OH)4 will rapidly react with itself
to form SiO2 (condensation reaction)
condensation of Al(OH)3 proceeds slowly Si(OH)4
? SiO2 2 H2O 2Al(OH)3 ? Al2O3 3 H2O or
Al(OH)3 ? AlOOH H2O notice that as clays
are weathered, ions are liberated to the
soil clays neutralize acidic precipitation ?
acids speed up weathering
7
Carbonates
The most abundant carbonate is calcium
carbonate (limestone, calcite) Low solubility in
water, Ksp 4.5x10-9 increases pH of water
(basic) Neutralizes acidic rain Weathers rapidly
under acidic conditions Important in the
buffering of pH due to reaction with
H2CO3 atmosphere CO2 ocean HCO3- crus
t CO32-
8
Soils
Inorganic soil component Organic soil component
diameter particle type humic
substances plants atoms 0.1 0.3
nm fungi clusters 0.5 2 nm microbes nano
1-100 nm dead leaves, grass clay lt 5000 nm (
lt 5 ?m) wood silt 5 74 ?m sand 74 ?m 4.75
mm various degrees of decay aerobic/anaero
bic pebbles, stones, boulders also, water,
salts, and gases
9
Organic Acids in Soil
As dead plants, animals, and waste die/decompose,
they liberate organic molecules to soil
10
Role of Organic Acids in Soil
acidify the soil
-
pKa 3-5
complexation of ions in the soil