Phases of matter: Comparison - PowerPoint PPT Presentation
Title:
Phases of matter: Comparison
Description:
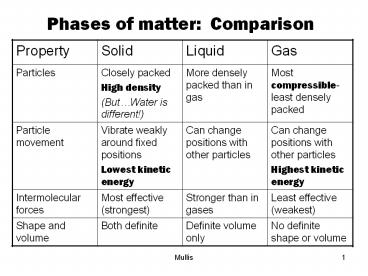
Phases of matter: Comparison Gas Liquid Solid Property Most compressible-least densely packed More densely packed than in gas Closely packed High density – PowerPoint PPT presentation
Number of Views:106
Avg rating:3.0/5.0
Title: Phases of matter: Comparison
1
Phases of matter Comparison
Property Solid Liquid Gas
Particles Closely packed High density (ButWater is different!) More densely packed than in gas Most compressible-least densely packed
Particle movement Vibrate weakly around fixed positions Lowest kinetic energy Can change positions with other particles Can change positions with other particles Highest kinetic energy
Intermolecular forces Most effective (strongest) Stronger than in gases Least effective (weakest)
Shape and volume Both definite Definite volume only No definite shape or volume
2
Surface tension
- Force that pulls adjacent parts of a liquid
surface together. - The higher the attractive forces between
particles in the liquid, the higher the surface
tension. - Hydrogen bonds make water have higher surface
tension than most liquids.
Soap
Water droplet
3
Solids
- Crystalline solids Particles are arranged in an
orderly, geometric, repeating pattern. - Examples Emerald, diamond, calcite
- Amorphous solids (Without shape)
- Particles are arranged randomly.
- Examples Glass, plastic
4
Changing states
- Equilibrium When there is no net change in a
system. - Dynamic equilibrium
- When a vapor is in equilibrium with its liquid as
one molecule leaves the liquid to become a vapor,
another molecule leaves the vapor to become a
liquid. In other words, an equal number of
molecules will be found moving in both
directions.
5
LeChâteliers Principle
- When a system at equilibrium is disturbed by
application of stress, it attains a new
equilibrium position that minimizes the stress. - "If stress is applied to a system at
equilibrium,the system will tend to readjust so
that the stress is reduced."
6
Boiling Point
- Vapor pressure Pressure exerted by a vapor
Pressure of the liquid at given temperature - Liquid boils when its vapor pressure equals
pressure of the atmosphere. - Boiling is the conversion of a liquid to vapor
within the liquid as well as at its surface. - Boiling point is the temperature at which the
equilibrium vapor pressure of the liquid equals
the atmospheric pressure. - Volatile liquids are liquids that evaporate
readily.
7
Boiling Point, cont.
- High elevation Low atmospheric pressure
- Low atmospheric pressure lower boiling point
- High pressure in pressure cooker increased
boiling point, faster cooking - If pressure above liquid increases, the liquid
temperature rises until it matches the new
pressure and boils again.
8
Separation by Distillation
- Distillation is the separation of liquid
substances according to their different boiling
points. - As a liquid mixture is heated, the substance with
the lower boiling point will vaporize first. - Distillate Condensed liquid substance
9
Kinetic Energy and Equilibrium Vapor Pressure
- In the beginning
- particles condensing to liquid phase
- particles evaporating to gas phase
- Increase temp Increase kinetic energy
- Now, more molecules have enough energy to leave
the liquid. - More vapor molecules higher vapor pressure
- Equilibrium will soon be established, but at a
higher vapor pressure.
10
Phase DiagramA phase diagram is a graph of
pressure vs. temperature that shows the
conditions under which phases of matter exist.
Critical temp (Tc) Above this, the substance
cannot exist in the liquid state.
11
Four major "points" on a phase diagram
- Triple point, TP - All three phases can exist in
equilibrium at this temperature and pressure. - (The solid-liquid line and the liquid-vapor line
meet.) - Normal boiling point, Tb - The temperature at
which the vapor pressure of a liquid is equal to
standard atmospheric pressure. - (Standard atmospheric pressure line crosses the
liquid-vapor line.) - Normal melting point, Tm - The temperature at
which the vapor pressure of the solid and the
vapor pressure of the liquid are equal. - (Standard atmospheric pressure line crosses
the solid-liquid line.) - Critical temperature, Tc - The temperature above
which no amount of pressure will liquefy a vapor.
- (The liquid-vapor line becomes vertical.)