Abstract - PowerPoint PPT Presentation
1 / 7
Title:
Abstract
Description:
Cooking the broccoli on the stove for five and ten minutes released the most ... Water was lost during the cooking of the broccoli in the stove and microwave. Results ... – PowerPoint PPT presentation
Number of Views:112
Avg rating:3.0/5.0
Title: Abstract
1
Abstract
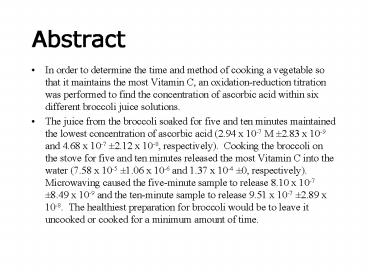
- In order to determine the time and method of
cooking a vegetable so that it maintains the most
Vitamin C, an oxidation-reduction titration was
performed to find the concentration of ascorbic
acid within six different broccoli juice
solutions. - The juice from the broccoli soaked for five and
ten minutes maintained the lowest concentration
of ascorbic acid (2.94 x 10-7 M 2.83 x 10-9 and
4.68 x 10-7 2.12 x 10-8, respectively). Cooking
the broccoli on the stove for five and ten
minutes released the most Vitamin C into the
water (7.58 x 10-5 1.06 x 10-6 and 1.37 x 10-4
0, respectively). Microwaving caused the
five-minute sample to release 8.10 x 10-7 8.49 x
10-9 and the ten-minute sample to release 9.51 x
10-7 2.89 x 10-8. The healthiest preparation
for broccoli would be to leave it uncooked or
cooked for a minimum amount of time.
2
Introduction
- The objectives
- To find the concentration of Vitamin C within
broccoli juice solution samples. - To determine the time and method of cooking
broccoli so that the vegetable maintains the most
ascorbic acid.
- The assumptions
- The broccoli juice contained the ascorbic acid
released during cooking therefore, the solutions
with lower concentrations of Vitamin C maintained
more ascorbic acid in the vegetable. - Soaked broccoli and broccoli sitting in the water
the least amount of time should retain the most
ascorbic acid.
3
Materials and Methods
125 mL Erlenmeyer flask Beakers Broccoli Buret
Dichlorophenol-indophenol Distilled
water Microwave Pure ascorbic acid Solid meta-p
hosphoric acid Stove Tap water Volumetric glass
kit Ziploc bags
Using parallel dilutions dilute the 5 x 10-3 M
DCIP solution to 4 x 10-5 M. Titrate the 20 mL
solution (10 mL broccoli juice and 10 mL
metaphosphoric acid) with the 4 x 10-5 M DCIP
solution until the solution turns a light pink.
4
Broccoli Juice Samples
Bought a head of broccoli from Frys grocery
store. Stored it in an airtight Ziploc bag in a r
efrigerator. Head of broccoli cut into six pieces
of similar weight (approximately 26.0000
grams). Pieces of broccoli cooked in 330 mL of ta
p water Boiled two pieces of broccoli on the sto
ve in an aluminum pot for 5 and 10 minutes.
Microwaved two pieces of broccoli for 5 and 10
minutes in a ceramic bowl. Soaked two pieces of b
roccoli in room-temperature water for 5 and 10
minutes in a glass jar. Broccoli removed from jui
ce and juice stored in clean plastic soda bottles
in a refrigerator until needed.
5
Observations
- Color of solutions ranged from lightly tinted
green to dark green. - When metaphosphoric acid was added, the broccoli
samples went colorless. - When the titrated solutions were allowed to sit
for 30 minutes or more, the color pink had
disappeared. - Water was lost during the cooking of the broccoli
in the stove and microwave.
6
Results
7
Further Work
- Other tests using broccoli juice solutions
- Titrating a solution of digested pieces of
broccoli instead of just the solutions in which
the broccoli was cooked. - Differing the amount of water used to see if the
amount of water used in cooking affects the
amount of ascorbic acid released into the water. - Titrating other vegetables using the methods
prescribed earlier in order to see whether they
lose ascorbic acid in relatively the same way as
broccoli.
- Recommendation for the previously specified
methods - Boiling/microwaving/ soaking broccoli for a
larger variety of times (such as samples cooked
for 5 minutes, 10 minutes, 15 minutes, and 20
minutes). - Allowing titrated solution to be a darker rose
color instead of a light pink.