Soil - PowerPoint PPT Presentation
1 / 41
Title:
Soil
Description:
have enough rain in summer so that the amount of stored moisture plus rainfall ... soil volume influenced by secretions of earthworms; 2-3 mm thickness - sites of ... – PowerPoint PPT presentation
Number of Views:234
Avg rating:3.0/5.0
Title: Soil
1
Soil
- What is it?
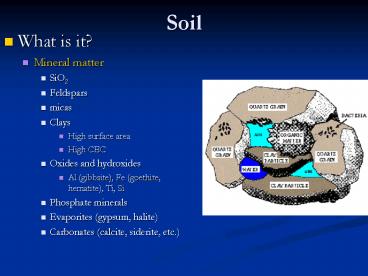
- Mineral matter
- SiO2
- Feldspars
- micas
- Clays
- High surface area
- High CEC
- Oxides and hydroxides
- Al (gibbsite), Fe (goethite, hematite), Ti, Si
- Phosphate minerals
- Evaporites (gypsum, halite)
- Carbonates (calcite, siderite, etc.)
2
Soil
- What is it? (cont.)
- Organic matter
- High CEC
- Source of organic acids
- Organisms
- Microfauna
- mesofauna
- Air
- Water
- Contains ions, organic matter, silica in solution
- Weathering
- Organic processes
- Rainfall/snowmelt wet deposition
- Dry deposition
3
Soil Moisture
- The volume of air and water in pore spaces is
complementary as one increase, the other
decreases. In poorly drained soils, all pore
space may be occupied by water. - In freely drained soils, water lost from large
cavities and larger pores is called gravitational
water. - About two or more days after raining or
irrigation, all gravitational water will be
removed, and the soil is said to be at field
capacity. - In this condition, water remains in finer pores,
known as capillary water, held by capillary
attraction to the surface of mineral particles. - Under drought conditions, even capillary water
can be removed such that plants can no longer
remain turgid. This point is called the wilting
point, and is dependent on the soil properties. - The amount of water that exists between the field
capacity and the wilting point is called the
available water capacity. - Water held in soil above the oven-dry temperature
of 105 is known as hygroscopic water and is
generally not available for plant use, as this
water is virtually part of the soil matrix
itself.
4
Soil Moisture
5
Soil Moisture Regimes
- Aquic moisture regime (Hydric)
- The aquic (L. aqua, water) moisture regime is a
reducing regime in a soil that is virtually free
of dissolved oxygen because it is saturated by
water. - Aridic and torric
- (L. aridus, dry, and L. torridus, hot and dry)
moisture regimes - occur in areas of arid climates.
- little or no leaching in this moisture regime,
and soluble salts accumulate in the soils if
there is a source. - Udic moisture regime
- (L. udus, humid)
- common to the soils of humid climates that have
well distributed rainfall - have enough rain in summer so that the amount of
stored moisture plus rainfall is approximately
equal to, or exceeds, the amount of
evapotranspiration - or have adequate winter rains to recharge the
soils and cool, foggy summers, as in coastal
areas. - Water moves downward through the soils at some
time in normal years.
6
Soil Moisture
- Ustic moisture regime
- ustic (L. ustus, burnt implying dryness)
- intermediate between the aridic regime and the
udic regime - Xeric moisture regime
- xeric (Gr. xeros, dry)
- Mediterranean climates
7
Organic Matter
- 0.5 to 5 in mineral soils
- Amorphous in nature
- Decomposition of plant and animal residue
- Major influence on physical/chemical properties
of soils
8
Organic Matter
- Nonhumic substances
- Compounds belonging to the well known classes of
biochemistry - amino acids
- carbohydrates
- lipids
- Humic substances
- Complex organic compounds with high-molecular
weight - Multiple rings, multiple polar substitutes
(Phenols, carboxylic acids, aliphatic hydroxides) - Many sites to chelate ions like Ca2, Fe2, Al3,
etc. - Yellow, brown to black substances formed by
secondary synthesis reactions
9
Organic Matter
- Sources
- Litter
- macroorganic matter that lies of the soil surface
- important in nutrient cycling in forest and
natural grasslands - Light fraction
- plant residues in various stages of decomposition
- labile portion
- source of nutrients for plants
- affected by pH, temp, texture, and moisture
- Microbial biomass
- soil bugs
- agent for plant residue decomposition
- labile pool of nutrients
- Water soluble organics
- soil solution
- low molecular weight alphatic and aromatic acids
- important in plant nutrition
- Soil Enzymes
- soil contains wide variety
10
Organic Matter
- Formation of Humic and Fulvic acids not well
understood - Most recent theory follows synthesis from
polyphenols
11
Soils and Organisms
12
Soil Organisms
13
Soil Organisms Microflora
- Nitrogen-fixing bacteria
- form symbiotic associations with the roots of
legumes like clover and lupine, and trees such as
alder and locust. - The plant supplies simple carbon compounds to the
bacteria, and the bacteria convert nitrogen (N2)
from air into a form the plant host can use. - When leaves or roots from the host plant
decompose, soil nitrogen increases in the
surrounding area. - Nitrifying bacteria
- change ammonium (NH4) to nitrite (NO2-) then to
nitrate (NO3-) - Denitrifying bacteria
- convert nitrate to nitrogen (N2) or nitrous oxide
(N2O) gas. - Denitrifiers are anaerobic, meaning they are
active where oxygen is absent, such as in
saturated soils or inside soil aggregates. - Actinomycetes
- bacteria that grow as hyphae like fungi
- responsible for the characteristically earthy
smell of freshly turned, healthy soil. - decompose a wide array of substrates, but are
especially important in degrading recalcitrant
(hard-to-decompose) compounds, such as chitin and
cellulose, - active at high pH levels.
14
Soil Organisms Microflora
- saprophytic fungi
- convert dead organic material into fungal
biomass, carbon dioxide (CO2), and small
molecules, such as organic acids. - generally use complex substrates, such as the
cellulose and lignin, in wood, - essential in decomposing the carbon ring
structures in some pollutants. - mycorrhizal fungi
- colonize plant roots.
- In exchange for carbon from the plant,
mycorrhizal fungi help solubolize phosphorus and
bring soil nutrients (phosphorus, nitrogen,
micronutrients, and perhaps water) to the plant.
15
Soil Organisms Microfauna
- Nematodes are non-segmented worms typically 1/500
of an inch (50 µm) in diameter and 1/20 of an
inch (1 mm) in length. - important in mineralizing, or releasing,
nutrients in plant-available forms. - When nematodes eat bacteria or fungi, ammonium
(NH4) is released because bacteria and fungi
contain much more nitrogen than the nematodes
require. - Protozoa
- feed primarily on bacteria, but also eat other
protozoa, soluble organic matter, and sometimes
fungi. - important role in mineralizing nutrients
- release the excess nitrogen in the form of
ammonium (NH4).
16
Soil Air
- Atmosphere penetrates into the soil through the
pore space and fissures. - Soil air differs from atmospheric air in that
- it is saturated with water vapor (near 100
humidity), and - carbon dioxide, a by-product of decomposition, is
sometimes 5-10 times higher. - When the pores are saturated with water, fresh
oxygen can not diffuse into the soil, creating
anaerobic conditions. - Plant growth is inhibited, and chemical reduction
may occur in soils (as opposed to oxidation). - The by-products of the reduction of nitrates,
manganese oxide, sulfate, and iron oxide cause
fermentation that produces gases in the soil that
are ultimately released to the atmosphere. - Such gases include nitrogen (N2), nitrous oxide
(NO2), hydrogen sulfide (H2S), carbon dioxide
(CO2), carbon monoxide (CO), and methane (CH4). - Disruption of natural soil processes, such as by
deforestation and increased cultivation, releases
carbon dioxide to the atmosphere.
17
Soil Formation
- Initial state
- Relief (Topography)
- Parent material
- Influxes
- Climate
- Temperature
- Precipitation
- Controls weathering reaction and soil biota
- Organisms
- Vegetation
- Animals
- Bacteria and fungi
- Time
18
Soil Systems
- Soil porosity
- Mediates chemical weathering moisture
retention, gas exchange - Controlled by both physical and chemical
processes - Physical transport
- Solid and dissolved matter is washed down through
soils - Capillary action and evaporation can lead to
upward transport of dissolved material - Frast action, root growth, tree falls, and
burrowing can mix material in a soil profile - Lateral porosity can be greater than vertical
- Chemical activities of many species (H2O, CO2,
O2)can change tremendously on geologic and short
term (minutes days) time scales
19
Soil Chemical Processes
- Formation of soil acidity
- Oxidation of organic matter
- Creation of acetates, Biogeochemical processes
- Bacterial degradation
- Formation of fulvic and humic acids
- Nitrogen fixing
- Ion exchange
- Soils contain abundant leachable ions and
molecules - Reactions take place on thin films (double layer)
and grain surfaces - Clays and organic matter have high CEC
20
Biogeochemical Processes
- detritusphere
- Association with plant litter
- Fungi decompose cellulose while taking oxygen in
and respiring carbon dioxide. - Inside anoxic corners of leaf structure, bacteria
convert nitric oxides to nitrogen. - driolosphere
- portion of soil volume influenced by secretions
of earthworms 2-3 mm thickness - sites of
enhanced C, N and P mineralization - Allow rainwater to penetrate soil
21
Biogeochemical Processes
- Porosphere
- between soil particles, aggregates, and roots
- aggregatusphere
- aggregates form when combination of microbial and
plant mucigels, fungal hyphae and small rootlets
bind multiple particles - bacteria attach to sand grains by fine fibrillae
extending from their cell walls and by
extracellular mucilaginous polysaccharides
(mucigels) - more intensively colonized by organisms and are
more active sites metabolically than soil as a
whole. C, N, S and P, sugars of microbial origin
gt than soil generally - nitrates (NO3-), ammonia (NH4), carbon dioxide
(CO2), nitric oxides - Rhizosphere
- Adjacent to roots and rootlets
- Area of extremely high metabolic activity and
chemical process
22
Soil Composition
23
Soil Color
- Soil colors attributed to soil attributes
24
Soil Horizons
- Soils may be divided into several horizons
- Manifestation of pedogenetic process and parent
material - Controlled by water movement
- Interaction between meteoric inputs and
vegetative canopy - Water transports ions, radicals, gases, particles
- Contributes to weathering of parent material
25
Soil Horizons
- There are 4 processes involved in horizon
differentiation - Additions to the soil
- water as precipitation, condensation, or runoff
- O2 and CO2 from the atmosphere
- N, Cl, and S from the atmosphere and
precipitation - organic matter from biotic activities
- material from sediments
- energy from the sun
- Losses from the soil
- water by evapotranspiration
- N by denitrification
- C as CO2 from oxidation of O.M.
- soil by erosion
- energy by radiation
- water and material in solution or suspension
26
Soil Horizons
- Translocations
- clay, organic matter, iron oxides, and chemicals
by water - nutrients circulated by plants
- soluble salts in water
- soils by animals
- Transformations
- decomposition of organic matter
- reduced particle size by weathering
- mineral transformations (primary to secondary)
- clay and organic matter reactions
27
Master Horizons
- designated by capital letters, such as O, A, E,
B, C, and R. - O horizons
- They are dominated by organic material.
- Some O layers consist of undecomposed or
partially decomposed litter, such as leaves,
twigs, moss, and lichens, that has been
decomposed on the surface they may be on the top
of either mineral or organic soils. - Other O layers, are organic materials that were
deposited in saturated environments and have
undergone decomposition. - The mineral fraction of these layers is small and
generally less than half the weight of the total
mass. - In the case of organic soils (peat, muck) they
may compose the entire soil profile. - Organic rich horizons which are formed by the
translocation of organic matter within the
mineral material are not designated as O
horizons.
28
Master Horizons
- A horizons
- Mineral horizons that formed at the surface or
below an O layer, - exhibit obliteration of all or much of the
original rock or depositional structure (in the
case of transported materials). - show one or more of the following
- An accumulation of humified organic matter
intimately mixed with the mineral fraction and
not dominated by characteristic properties of the
E or B horizons or, - Properties resulting from cultivation, pasturing
or other similar kinds of disturbance.
29
Master Horizons
- E horizons
- Eluviation layer
- Mineral horizons in which the main feature is
loss of silicate clay, iron, aluminum, or some
combination of these, leaving a concentration of
sand and silt particles and lighter colors. - The horizons exhibit obliteration of all of much
of the original rock structure.
30
Master Horizons
- B horizons
- Illuviation layer
- Horizons in which the dominant feature(s) is one
or more of the following - An illuvial concentration of silicate clay, iron,
aluminium, carbonates, gypsum, or humus - A residual concentration of oxides or silicate
clays, alone or mixed, that has formed by means
other than solution and removal of carbonates or
more soluble salts - Coatings of oxides adequate to give darker,
stronger, or redder colors than overlying and
underlying horizons but without apparent
illuviation of iron - An alteration of material from its original
condition that obliterates original rock
structure, that form silicate clay, liberates
oxides, or both, and that forms a granular,
blocky, or prismatic structure
31
Master Horizons
- C horizons
- Mineral horizons that are little altered by soil
forming processes. - They lack properties of O, A, E, or B horizons.
- The designation C is also used for saprolite,
sediments, or bedrock not hard enough to qualify
for R. - The material designated as C may be like or
unlike the material form the A, E, and B horizons
are thought to have formed.
32
Master Horizons
- R Horizon
- Consolidated bedrock (hard bedrock), such as
granite, basalt, quarzite, sandstone, or
limestone. - Small cracks, partially or totally filled with
soil material and occupied by roots, are
frequently present in the R layers.
33
Soil Horizons
- Soils may be divided into several horizons based
on composition and chemical process
34
(No Transcript)
35
(No Transcript)
36
Soil Taxonomy
- There are 12 general soil orders
- Based on soil characteristics
- Loosely correlated with latitude (climate zones)
37
(No Transcript)
38
(No Transcript)
39
(No Transcript)
40
- Profile 1 is a hydric soil and the wettest of
the soils in this photo series. It shows two
hydric soil characteristics, a thickened organic
layer, explained below, and a gray matrix
explained under the second profile. Profile 1
occurs at the lowest point in the wetland and is
inundated for extended periods of time. The
ponded water fills the soil pores preventing air
from entering the soil. A few days of soil
saturation is usually sufficient for soil
microbes to exhaust the supply of dissolved
oxygen in the soil water. The lack of oxygen
slaws the process of microbial decomposition
causing partially decomposed organic matter to
accumulate above the mineral layers of soil
creating the thickened 0 and A layers apparent in
this soil profile. The thick organic layer at the
soil surface indicates a hydric soil. - Profile 2, also a hydric soil, is somewhat
higher in the landscape and although still
subject to fluctuating water rabies and lengthy
periods of saturation, is not inundated for long
periods. Therefore, the soil shows the gray
matrix color in the Band C layers, but not the
thickened organic surface. Without oxygen,
microbes must utilize iron compounds to obtain
energy from organic matter. In the process, iron
compounds are converted from insoluble to soluble
and flushed out leaving the gray background
color. As iron precipitates in the aerated zones,
additional soluble iron migrates from anaerobic
sites unitl they become so depleted of iron that
the gray color predominates. The term matrix is
used to describe conditions in the dominant
volume of soil within a layer. A soil layer is
considered to be anaerobic when the sail matrix
is dominated by gray depletion color. Layers B
and C show the gray matrix indicating that these
layers are saturated for long periods. The dull
gray matrix extending to the soil surface
indicates a hydric soil. - Profile 3 is also a hydrie soil and although
saturated far shorter periods of time, still
shows the strong gray matrix color all the way to
the soil surface. Fluctuating water levels in the
soil allow air to fill the larger pores as the
water level lowers and then trap the air as the
water level rises again. When the iron dissolved
in the water encounters a zone of trapped air, it
farms a strong, red-brown colored precipitate.
The precipitated colors are known as
concentrations or mottles and are evident here in
the AB and B layers. The gray matrix with bright
mottles extending to very near the soil surface
indicates a hydric soil.
41
- Profile 4 is significantly higher in the
landscape and though it weakly displays wetness
characteristics it is not a hydric soil. In both
the A and B layers the matrix has medium colors
associated with the original evenly distributed
oxidized iron coatings on sand grains. Small
areas of iron depletion and concentration exist,
however the segregation process and attendant
gray color is not dominant in any layer. This
soil is probably saturated to the surface for
only very short periods of time and is,
therefore, not a hydric soil. - Profile 5 is not a hydric soil and shows
evidence of saturation only in the deeper layers
of the soil. As stated previously, iron
depletions and concentrations are evidence of
iron segregation due to saturation. The AB layer
is saturated and anaerobic so infrequently that
gray depletions are almost nonexistent. The
deeper B layer shows faint depletions indicating
that the soil is saturated only at depth and only
for relatively short periods. Since the sail is
seldom saturated to the surface and only
saturated at depth for short periods of time, it
is not a hydric soil. - Profile 6 occupying the highest landscape
position in this series, shows essentially no
evidence of saturation and is not a hydrie soil.
In this soil.the vertical redistribution of iron
into horizons is associated with water
percolating down through the soil profile. As a
result, iron is evenly distributed within each of
the soil layers. Saturation is either absent or
restricted to such brief periods that the soil
dues not develop anaerobic conditions, thus
preventing iron segregation and development of
the gray matrix within the soil horizons. The
absence of inundation also prevents the
development of organic accumulations on the
surface.