Periodic Table - PowerPoint PPT Presentation
1 / 28
Title:
Periodic Table
Description:
Traditionally designated by a Roman numeral and a letter (either A or B) at the ... High thermal/electrical conductivity, ductility, malleability, metallic luster. ... – PowerPoint PPT presentation
Number of Views:64
Avg rating:3.0/5.0
Title: Periodic Table
1
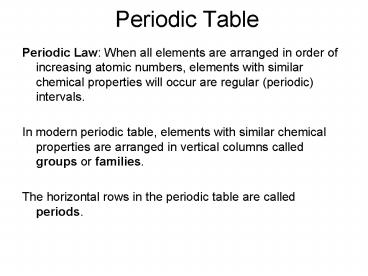
Periodic Table
- Periodic Law When all elements are arranged in
order of increasing atomic numbers, elements with
similar chemical properties will occur are
regular (periodic) intervals. - In modern periodic table, elements with similar
chemical properties are arranged in vertical
columns called groups or families. - The horizontal rows in the periodic table are
called periods.
2
(No Transcript)
3
Groups and Periods
- Periodic table group or family
- Traditionally designated by a Roman numeral and a
letter (either A or B) at the top of the column. - Designated only by a number from 1 to 18 in a
modern but as yet not universally-used
designation. - Periodic table Period
- Periods are numbered numerically from top to
bottom of the periodic table. - In a modern table, elements 58-71 and 90-103 are
not placed in their correct periods, but are
located below the main table.
4
(No Transcript)
5
Electronic arrangements in Atoms
- Rutherfords atom
- Solar system model
- Niels Bohr proposed that the electron in hydrogen
atom could occupy orbits only at specific
distances from the nucleus. In other words, the
electron moved in any one of a series of circular
orbits around the nucleus.
Electron can change orbits only by absorbing or
releasing energy, with higher energy orbit
located farther from the nucleus.
6
Quantum Mechanical model of atom
- According to the quantum mechanical model of
electron behavior, the precise paths of electrons
moving around the nucleus cannot be determined
accurately. - Instead of circular orbits, the location and
energy of electrons moving around the nucleus is
specified using the three terms shell, subshell
and orbital. - Locate your position on map 1
- Locate your position on map 2
- Locate your position on map 3
7
Shell
- The location of electrons in a shell is indicated
by assigning a number n to the shell and all
electrons located in the shell. - The value of n is a whole number- 1, 2, 3, 4 etc.
- The higher the n value,
- the higher is the energy of the shell
- the higher is the contained electrons
- the greater is the distance from the nucleus
8
Subshell
- Each shell is made up of one or more subshells
that are designated by a letter from the group s,
p, d, or f. - The number of the shell to which a subshell
belongs is combined with the letter of the
subshell to clearly identify subshells. - For example, a p subshell located in the third
shell (n 3) would be disignated as a 3p
subshell. - The number of subshells located in a shell is the
same as the number of the shell. Thus, shell
number 3 (n 3) contains three subshells,
designated 3s, 3p, and 3d. - Electrons located in a subshell are often
identified by using the same designation as the
subshell they occupy. Thus electrons in a 3d
subshell are called 3d electrons.
9
Atomic Orbitals
- The description of the location and energy of an
electron moving around a nucleus is completed in
the quantum mechanical model by specifying an
atomic orbital in which the electron is located. - Each subshell consists of one or more atomic
orbitals, which are specific volumes of space
around the nucleus in which electrons move. - Atomic orbitals are designated by the same number
and letter used to designate the subshell to
which they belong. Thus, an s orbital located in
a 2s subshell would be called a 2s orbital.
10
Atomic Orbitals
- All s subshells consist of a single s orbital.
- All p subshells consist of three p orbitals.
- All d subshells consist of five d orbitals.
- All f subshells consist of seven f orbitals.
- According to the quantum mechanical model, all
types of atomic orbitals can contain a maximum of
two electrons. - Thus, a single d orbital can contain a maximum of
2 electrons, and a d subshell that contains seven
d orbitals can contain a maximum of 14 electrons.
11
Atomic Orbitals
- Atomic Orbital shapes
- The shape of the orbital should not be confused
to mean that the electrons move around the
orbital shape surface. It only determines the
probability to find the electron in a specific
location.
12
Energy of electrons
- The energy of electrons around a nucleus depends
on two factors. - Electron energy increases with increasing n
value. Thus an electron in the third shell (n
3) has more energy than an electron in the first
shell (n 1). - For equal n values but different orbitals, the
energy of electrons in orbitals increases in the
order s, p, d and f. Thus, a 4p electron has more
energy than a 4s electron. - Example 3.3
13
Shells, Subshells, orbitals electrons
14
Shell model and chemical properties
- The outermost occupied shell (with the highest n
value) is called as the valence shell. - Atoms with the same number of electrons in the
valence shell have similar chemical properties. - Example 3.4
15
(No Transcript)
16
Electronic Configurations
- Electronic configurations give details of the
arrangements of electrons in atoms. - The notation used to represent electronic
configurations is 1s22s22p6 etc., where the
occupied subshells are indicated by their
identifying number and letter such as 2s, and the
number of electrons in the subshell is indicated
by the superscript on the letter. Thus, in the
example above, the 2s2 notation indicates that
the 2s subshell contains two electrons. - Electrons will fill subshells in the order of
increasing energy of the subshells. Thus, a 1s
subshell will fill before a 2s subshell. - The order of subshell filling must obey Hund's
rule and the Pauli exclusion principle.
17
Electronic Configurations
- Hunds rule-
- According to Hund's rule, electrons will not join
other electrons in an orbital of a subshell if an
empty orbital of the same energy is available in
the subshell. - Thus, the second electron entering a p subshell
will go into an empty p orbital of the subshell
rather than into the orbital that already
contains an electron. - The Pauli exclusion principle-
- Electrons behave as if they spin on an axis.
- According to the Pauli exclusion principle, only
electrons spinning in opposite directions can
occupy the same orbital within a subshell. - Combined- Electrons will pair with other
electrons in an orbital only if there is no empty
orbital of the same energy available and if there
is one electron with opposite spin already in the
orbital.
18
Electronic Configurations
- Example 3.6 a, b
19
- Relative energies and electron-filling order for
shells and subshells
Some low energy subshells of a specific shell
have energies lower than the upper subshell of a
preceding shell.
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s,
4f, 5d, 6p, 7s, 5f, 6d, 7p
20
Electronic Configurations
- Electronic configuration for
- An atom that contains 17 electrons (Example 3.6)
- An atom that contains 23 protons (Learning Check
3.6)
21
Electronic Configurations
- Electronic Configuration gives information about
the shells, subhsells and orbitals. But are
cumbersome. Hence, use - Noble Gas configuration An electronic
configuration in which the last eight electrons
occupy and fill the s and p subshells of the
highest-occupied shell. - Noble gas configurations can be used to write
electronic configurations in an abbreviated form
in which the noble gas symbol enclosed in
brackets is used to represent all electrons found
in the noble gas configuration. - Sodium Ne3s1. The symbol Ne represents the
electronic configuration of neon, 1s22s22p6. - Magnesium
- An atom that contains 17 electrons
- An atom that contains 23 protons
Ne3s2. The symbol Ne represents the
electronic configuration of neon, 1s22s22p6.
22
Classification according to Distinguishing
Electrons
The distinguishing electron is the last electron
listed in the electronic configuration of the
element.
23
Representative, Transition, Inner-transition,
Noble Gases
Noble Gases Groups VIII A filled s and p
subshells (exception He) Representative elements
s and p areas (other than VIII A)
Transition elements d area Inner-transition
elements f area
24
Metals, Nonmetals Metalloids
Metals- elements in the left two-thirds High
thermal/electrical conductivity, ductility,
malleability, metallic luster.
Metalloids- elements narrow diagonal band between
metals and nonmetals Some characteristics of each
Nonmetals- elements in the right
one-third Brittle, powdery solids or gases
25
Periodic Trends
- Trends in Metallic Properties
- Elements in the same period of the periodic table
become less metallic and more nonmetallic from
left to right across the period. - Elements in the same group of the periodic table
become more metallic and less nonmetallic from
top to bottom down the group. - Trends in the size of atoms
- For representative elements in the same period,
atomic size decreases from left to right in the
period - For representative elements in the same group,
atomic size increases from top to bottom down the
group.
26
Metallic and size of atoms trends for
representative elements
27
Periodic Trends
- Ionization Energy of an element is the energy
required to remove an electron from an atom of
the element in the gaseous state. - This results in formation of a charged species
called ion. - First ionization energy is the energy to remove
the first electron from a neutral atom.
- Trends in Ionization Energy
- For representative elements in the same period,
the general trend is an increase from left to
right across the period. - For representative elements in the same group,
the general trend is a decrease from top to
bottom down the group.
28
Colors of elements
- Form a group with 3 other students having
different color cards - Green, Blue, Yellow, Pink
- Choose an element belonging to your color
- Describe the elements properties in terms of
- Metal/Nonmetal/Metalloid
- Size of atom (if possible)
- Representative, Transition, Inner-transition,
Noble gas - Write an abbreviated electronic configuration
(inner transition folks may skip this)