The Gas Laws - PowerPoint PPT Presentation
1 / 24
Title:
The Gas Laws
Description:
I. Kinetic Theory. gases. particles. motion. forces -actual gases don't ... A. Gas particles do not attract or ... jagged ends. The Gas Laws. III. Charles's Law ... – PowerPoint PPT presentation
Number of Views:106
Avg rating:3.0/5.0
Title: The Gas Laws
1
The Gas Laws
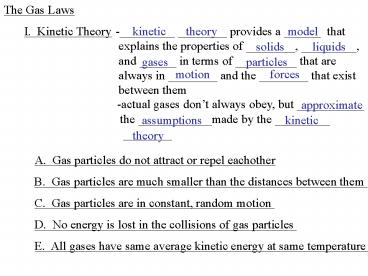
-_________ ________ provides a ______ that
explains the properties of ________, _________,
and ______ in terms of __________ that are
always in ________ and the ________ that exist
between them
kinetic
theory
model
I. Kinetic Theory
solids
liquids
gases
particles
motion
forces
-actual gases dont always obey, but ___________
the ____________made by the _________ ________
approximate
assumptions
kinetic
theory
A. Gas particles do not attract or repel
eachother
B. Gas particles are much smaller than the
distances between them
C. Gas particles are in constant, random motion
D. No energy is lost in the collisions of gas
particles
E. All gases have same average kinetic energy at
same temperature
2
The Gas Laws
II. Boyles Law
-the _______ of a gas _________ _________ with
the _________, providing the ___________ is held
________
volume
varies
inversely
pressure
temperature
constant
P1
V2
___
___
P1V1
P2V2
or
P2
V1
If a sample of Argon gas occupies a volume of
10.0 liters at a pressure of 200 kilopascals, at
what pressure would the same sample of gas
occupy 5.0 liters, if the temperature remains
constant?
Robert Boyle (1627-1691)
P1
V2
___
___
P2
V1
200 kPa
5.0 L
______
____
P2
10.0 L
P2
400 kPa
3
The Gas Laws
II. Boyles Law
If a sample of Carbon dioxide gas occupies a
volume of 35.0 liters at a pressure of 1.10
atmospheres, what volume would the same sample of
gas occupy at 1.25 atmospheres, if the
temperature remains constant?
Boyles Vacuum Chamber, Designed by Robert Hooke
P1
V2
___
___
P2
V1
1.10 atm
V2
_____
_______
1.25 atm
35.0 L
V2
30.8 L
4
The Gas Laws
III. Charless Law
-the _______ of a gas _________ _________ with
the ___________, providing the _________ is held
________
volume
varies
directly
temperature
pressure
constant
V1
T1
___
___
V1T2
V2T1
or
V2
T2
If a sample of Chlorine gas occupies a volume of
250 milliliters at a temperature of 22C, what
volume would the same sample of gas occupy at
-22C, if the pressure remains constant?
We need a temperature scale with no zero, and
no negative values!
Jacques Charles (1746-1823)
V1
T1
___
___
V2
T2
C
K
273
250 mL
250 mL
295 K
22C
______
_____
______
____
V2
V2
251 K
-22C
V2
V2
213 mL
-250 mL?
A negative volume?
5
The Gas Laws
III. Charless Law
1. Hypothesis What is the relationship between
volume and temperature
of a gas?
2. Prediction
3. Gather Data
A. Safety
1. Be careful not to drip or splash hot
vegetable oil on yourself. Goggles
mandatory, aprons recommended.
2. Capillary tubes break easily. Be cautious of
broken, jagged ends.
6
The Gas Laws
III. Charless Law
3. Gather Data
B. Procedure
1. Assemble thermometer/capillary tube apparatus
by carefully wrapping rubber bands around
both to hold the capillary tube to the
thermometer, arranging the open end toward
the bulb of the thermometer, just short of the
end.
7
The Gas Laws
III. Charless Law
3. Gather Data
B. Procedure
1. Assemble thermometer/capillary tube apparatus
by carefully wrapping rubber bands around
both to hold the capillary tube to the
thermometer, arranging the open end toward
the bulb of the thermometer, just short of the
end.
8
The Gas Laws
III. Charless Law
3. Gather Data
B. Procedure
2. Immerse the capillary tube on the
thermometer completely under the surface of
the hot oil bath. Allow the temperature on
your thermometer to reach 140C. Then, lift
the thermometer from the oil bath, pausing for
5 seconds to allow oil to rise up into the
tube.
3. Carry the tube/thermometer assembly back to
your lab table, being careful to catch the
drips of hot oil on a paper towel. Lay the
thermometer on a sheet of paper towel. Mark
the position of the oil plug. Take note of the
temperature. As the air in the tube cools,
mark the position of the oil plug as it
moves along the tube on the
9
The Gas Laws
III. Charless Law
3. Gather Data
B. Procedure
3. paper towel. With each mark on the paper
towel recording the position of the oil
plug, record the temperature at the time the
mark was made. Take as many
position/temperature readings as possible, until
the thermometer reads room temperature (about
23C).
10
The Gas Laws
III. Charless Law
3. Gather Data
B. Procedure
3. paper towel. With each mark on the paper
towel recording the position of the oil
plug, record the temperature at the time the
mark was made. Take as many
position/temperature readings as possible, until
the thermometer reads room temperature (about
23C).
4. Plot data on Excel spreadsheet and graph
results.
11
The Gas Laws
III. Charless Law
Temperature (in C)
Length (in mm)
4. Analyze Data
12
The Gas Laws
III. Charless Law
Temperature (in C)
Length (in mm)
4. Analyze Data
5. Draw Conclusions
13
The Gas Laws
III. Charless Law
-_________ _____ is the _______ possible
theoretical temperature, equal to _________, and
is the ___________ at which the ________ of a
sample of gas is _____, and all ________,
__________ movement of particles ________
absolute
zero
lowest
-273.15C
volume
temperature
zero
random
Brownian
ceases
-the coldest _______ temperature is _______, or
_________, the temperature of ______ _______
actual
4.22 K
William Thomson (Lord Kelvin) (1824-1907)
-268.78C
liquid
Helium
14
The Gas Laws
III. Charless Law
If a sample of methane gas occupies a volume of
14.75 liters at a temperature of 68F, at what
temperature, in F, would the same sample of gas
occupy 16.00 liters, if the pressure remains
constant?
Charless Hydrogen-filled balloon over Paris, 1783
V1
F
T1
9/5
C
32
___
___
V2
T2
C
K
273
14.75 L
293 K
______
_____
16.00 L
T2
T2
318 K
T2
113F
15
The Gas Laws
IV. Gay-Lussacs Law
-the _______ of a gas _________ _________ with
the ___________, providing the _________ is held
________
pressure
varies
directly
temperature
volume
constant
P1
T1
___
___
P1T2
P2T1
or
P2
T2
If the pressure in a propane tank is 965 mm Hg at
a temperature of 25C, what would the pressure in
the tank be at 50C, if the volume remains
constant?
Joseph Gay-Lussac (1778-1850)
P1
T1
___
___
P2
T2
C
K
273
965 mm Hg
298 K
_________
_____
P2
323 K
P2
1040 mm Hg
16
The Gas Laws
IV. Gay-Lussacs Law
If the air pressure in an autoclave is 833 torr
at a temperature of 212F, at what temperature,
in F, would the air pressure be 900 torr, if the
volume remains constant?
Gay-Lussac and Jean-Baptiste Biot in Hydrogen
balloon, 1804
P1
F
T1
9/5
C
32
___
___
P2
T2
K
C
273
373 K
833 torr
______
_____
900 torr
T2
T2
403 K
T2
266F
17
The Gas Laws
V. Combined Gas Law
-the _______ of a gas is _________ proportional
to ________ and directly proportional to
___________, and ________ is __________
proportional to ____________
pressure
inversely
volume
P1V1
P2V2
temperature
_____
_____
T1
T2
directly
volume
temperature
If the volume of a sample of Hydrogen sulfide gas
is 2.00 liters at a pressure of 110 kilopascals
and a temperature of 30.0C, what is the volume
of the same sample of Hydrogen sulfide, in
liters, at a temperature of 80.0C and a pressure
of 440 kilopascals?
(440 kPa)
P1V1
P2V2
(110 kPa)
V2
(2.00 L)
_____
_____________
__________
_____
303.0 K
353.0 K
T1
T2
V2
0.582 L
C
K
273
18
The Gas Laws
V. Combined Gas Law
If a Helium-filled balloon at sea level has a
volume of 2.1 liters at a pressure of 0.998
atmospheres and a temperature of 36C, and it is
released and rises to an elevation at which the
temperature is 28C and the pressure is 0.900
atmospheres, what will be the new volume of the
balloon?
(0.900 atm)
P1V1
P2V2
V2
(2.1 L)
(0.998 atm)
_____
_____
_____________
__________
T1
T2
309 K
301 K
V2
2.3 L
C
K
273
19
The Gas Laws
VI. Avogadros Principle
-______ ________ of gases at the same
____________ and _________ contain ______
numbers of ________
equal
volumes
temperature
pressure
Amadeo Avogadro (1776-1856)
particles
equal
-one ______ of gas contains __________ particles
and occupies _______ at _________ temperature
and pressure, which is ______ and ________
mole
6.02 x 1023
22.4 L
standard
0.00C
1.00 atm
How many molecules in 3.73 liters of ozone at STP?
1 mole O3
6.02 x 1023 molecules O3
3.73 L O3
x
___________
1.00 x 1023 molecules O3
x
_________________
22.4 L O3
1 mole O3
conversion factor
20
The Gas Laws
VI. Avogadros Principle
What is the volume, in liters, of 0.881 moles of
Fluorine gas at STP?
22.4 L F2
0.881 mole F2
x
________
19.7 L F2
1 mole F2
How many moles of Nitrogen gas are in 2.00 liters
at STP?
1 mole N2
2.00 L N2
x
________
0.0893 moles N2
22.4 L N2
What is the volume, in liters, of 5.0 kilograms
of methane at STP?
1000 grams CH4
1 mole CH4
22.4 L CH4
5.0 kg CH4
x
____________
7.0 x 103 L CH4
x
______________
x
_________
1 kg CH4
16.043 grams CH4
1 mole CH4
conversion factor
What mass, in grams, of Nitrogen dioxide occupies
15.50 liters at STP?
1 mole NO2
46.005 grams NO2
15.50 L NO2
x
__________
31.8 g NO2
x
______________
22.4 L NO2
1 mole NO2
21
The Gas Laws
VII. Ideal Gas Law
-___________ Law, ________ Law, ________ Law,
and _____________ Law can be combined into one
mathematical statement called the
______________, which describes the relationship
among _________, ________, ____________, and the
number of ______ of a gas
Avogadros
Boyles
Charless
Gay-Lussacs
Ideal Gas Law
pressure
volume
temperature
moles
ideal
real
-______ gases, as opposed to ______ gases, is
one in which the gas __________ take up no
______ and have no _______________ forces
between them, and so obey all of the gas laws
under all conditions
particles
space
intermolecular
-______ gases obey the gas laws, except at
extreme conditions, like very ______ _______ and
very ____ ____________, or when the gas is very
______, like water, or when _________ are very
______, like butane
real
high
pressure
low
temperature
polar
particles
large
22
The Gas Laws
VII. Ideal Gas Law
nRT
PV
R ideal gas constant 0.0821 Latm
molK
How many moles of Hydrogen gas are contained in a
vessel with a volume of 3.0 liters at a
temperature of 300 K and a pressure of 1.50
atmospheres?
nRT
(0.0821 Latm) molK
(1.50 atm)
(300K)
n
PV
(3.0 L)
R 0.0821 Latm molK
n
0.18 moles
At what Celsius temperature would 2.49 moles of
Helium gas occupy 1.00 liters if the pressure was
143 kPa?
nRT
(8.314 LkPa) molK
(143 kPa)
T
2.49 moles
PV
(1.00 L)
R 8.314 LkPa molK
T
6.91 K
-266.09 C
23
The Gas Laws
VII. Ideal Gas Law
MP
D
M molar mass of the gas
RT
What is the density of Carbon dioxide at standard
temperature and pressure?
MP
(44.009 g/mole)
D
D
(1.00 atm)
___________________
RT
(273.00 K)
(0.0821 Latm) molK
D
1.96 gatmmolK
1.96 g/L
molLatmK
What is the molar mass of a gas that has a
density of 1.09 g/L at a pressure of 1.02 atm and
a temperature of 25C? Which gas is it?
(1.02 atm)
M
MP
1.09 g/L
D
___________________
RT
(298 K)
(0.0821 Latm) molK
M
26.1 gLatmK
26.1 g/mol
C2H2 ethyne (acetylene)
LmolKatm
24
The Gas Laws
VIII. Gas Stoichiometry
What volume, in liters, of oxygen is required for
the complete combustion of 4.00 liters of propane
gas, if the pressure and temperature remain
constant?
1C3H8
3CO2
4H2O
5O2
5 liters O2
4.00 liters C3H8
x
_________
20.0 L O2
1 liter C3H8
Avogadros Law says that a mole of any gas under
the same conditions of temperature and pressure
occupy the same volume, so the mole ratio is a
volume ratio, too.
What mass, in grams, of Ammonium nitrate is
required to produce 0.100 liters of Dinitrogen
monoxide at STP?
1NH4NO3
1N2O
2H2O
1 mole N2O
1 mole NH4NO3
80.043 g NH4NO3
0.100 liters N2O
x
_________
0.357 g NH4NO3
x
_________
x
____________
22.4 liters N2O
1 mole N2O
1 mole NH4NO3