Cellular Respiration C6H12O6 602 6CO2 6H2O - PowerPoint PPT Presentation
1 / 58
Title:
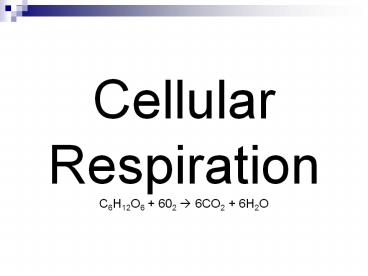
Cellular Respiration C6H12O6 602 6CO2 6H2O
Description:
The electrons are frequently accompanied by a hydrogen proton. ... FADH2 caries e- later down the chain and carries lower energy e- s ... – PowerPoint PPT presentation
Number of Views:737
Avg rating:3.0/5.0
Title: Cellular Respiration C6H12O6 602 6CO2 6H2O
1
Cellular RespirationC6H12O6 602 ? 6CO2 6H2O
2
Oxidation-reduction
- Oxidation-reduction LEO the lion goes GER.
- The electrons are frequently accompanied by a
hydrogen proton.
3
(No Transcript)
4
- Since electrons represent energy, oxidation
involves the release of energy - Reduction stores energy in the atom or molecule.
- Whenever one substance is oxidized, another
substance is reduced. - Oxidation-reduction reactions transfer energy
within living organisms.
5
ATP
- ATP ATP is the universal energy currency of all
cells. - High-energy covalent bonds link the three
phosphate groups. - When one of those bonds is broken, ADP and an
inorganic phosphate are left, and 7.3 kcal/mole
of energy are released.
6
- This is enough energy to drive endergonic
reactions in the cell or perform other work. - ATP is constantly being synthesized and broken
down in living cells - It cycles and recycles between ADP Pi and ATP.
- The energy required to synthesize ATP from ADP
Pi comes from photosynthesis or cellular
respiration.
7
Structure of ATP
- Ribose sugar bound to adenine base and chain of
three phosphate groups - Linked phosphates store energy of their
electrostatic repulsion - Phosphate transfer (phosphorylation) charges that
molecule
8
(No Transcript)
9
(No Transcript)
10
Step Wise Chemical Pathways
- The oxidation of glucose is a step wise reaction.
Complete oxidation occurs in 3 pathways
glycolysis, Krebs cycle, and the electron
transport chain. - NAD and FAD serve as electron carriers, and are
reduced and then later oxidized to keep the
reaction going.
11
NAD and FAD
- FADflavin adenine dinucleotide NAD
Nicotinamide adenine dinucleotide - (N 1 and 5 carry H to make FADH2 )
12
(No Transcript)
13
ALL CELLS perform Glycolysis
- Takes place in the CYTOPLASM
- It produces 4 ATP costs 2 ATP to get started, so
it nets 2 ATP for the cell - 10 enzyme-catalyzed steps
- First 5 devoted to splitting 6-C glucose into TWO
3-C G3P
14
Glycolysis
- Does NOT require oxygen
- Its end-product is pyruvate
- With oxygen present aerobic the pyruvate is
modified and goes into the Krebs Cycle aka
Citric Acid Cycle or TCA IF MITOCHONDRIA ARE
PRESENT!!
15
GLYCOLYSIS
- WITHOUT oxygen present anaerobic
- In organisms like yeast, fermentation takes place
and the end-product is ethanol - In organisms like us, deep in muscle tissue,
anaerobic respiration takes place as well, but
the end-product is lactic acid! - This causes muscle cramps once youve used up
your oxygen supply in the tissues!
16
GLYCOLYSIS
- Energy from glucose extracted in 10 steps
- Produces ATP by substrate level phosphorylation
- Only 3.5 efficientmost of the energy is still
tied up in the end-product, pyruvate
17
Glycolysis
- Glucose priming
- Change glucose into a compound that is readily
cleaved in half - Uses TWO ATP
- Cleavage and Rearrangement
- One molecule is G3P, the other must become G3P
18
(No Transcript)
19
Glycolysis
- Substrate-Level Phosphorylation and oxyidation.
- Thats what we call the remaining 5 reactions.
- Oxidation
- TWO e- and one proton H transferred from G3P
to NAD - Forms NADH
- One per G3P, thus 2 per glucose since this is
happening twice!
20
Glycolysis
- ATP generation by substrate level
phosphorlyation. - G3P is converted into pyruvate 2/glucose
- TWO ATP are made per G3P 4/glucose
- Tally 2 ATP net, 2 NADH important later, 2
pyruvate still house an awful lot of energy!if
you have mitochondria, you can harvest more
energy from pyruvate
21
(No Transcript)
22
Net Energy
- 24 kcal/ mole of glucose
- Puny but true. Life on this planet survived
about a billion years on just glycolysis
23
Recap
- All cells use glycolysisno O2 required
- Among first to evolve
- Occurs in cytoplasm
- Glucose ? 2 pyruvate
- 2 ADP ? 2 ATP
- 2 NAD ? 2 NADH
24
Recycling NAD
- Cell will accumulate NADH and run out of NAD
- Two ways to recycle
- FERMENTATION anaerobic
- RESPIRATION aerobic
25
Aerobic Respiration
- Oxygen is the final electron acceptor
- Water is the final product
- Cell must have mitochondria
- Pyruvate ? acetyl- CoA? Krebs cycle? ETS
26
Fermentation
- Organic molecules serve as final electron
acceptor - Occurs in many organisms EVEN THOSE CAPABLE OF
AEROBIC RESPIRATIONlike you! - Reduces all or part of the pyruvate
- Ethanol lactic acid are the most common end
products.
27
(No Transcript)
28
Aerobic Respiration
- Eukaryotes? occurs ONLY in mitochondria
- In prokaryotes it occurs along the plasma
membrane - Pyruvate is oxidized ?acetyl-CoA
- Oxidation of acetyl-CoA takes place in the Krebs
cycle
29
Producing Acetyl-CoA
- One C is cleaved from 3-C pyruvate and LEAVES AS
CO2 - Decarboxylation reaction
- The 2-C fragment is an acetyl group
- A PAIR of e- and an associated H reduces NAD to
NADH
30
(No Transcript)
31
USING ACETYL-CoA
- If it is NOT stored then it enters the Krebs
Cycle - Nine reactions in 2 stages
- Priming
- Acetyl CoA first joins the cycle
- Chemical groups are rearranged isomerization
step
32
- Energy Extraction is stage 2
- Four of the six reactions are oxidations,
electrons are removed and electron carriers
become VERY important in the production of ATP - One reaction generates an ATP via substrate level
phosphorylation
33
Reaction 1A condensation rxn.
- 2-C acetyl-CoA 4-C oxaloacetate ? 6-C
citrate CoA which can be used over and over! - Irreversible
- Inhibited by large amounts of ATP already present
34
Reaction 2 3--Isomerization
- Hydroxyl group repositioned
- Water removed from one C and then added to a
different C - RESULT a change in position of an H and OH
- Molecule is now ISOCITRATE
35
Reaction 4the first oxidation
- Isocitrate undergoes oxidative decarboxylationfan
cy for chopping off a carbon and losing a pair of
e-s in the process - The pair of e- reduce NAD to NADH
- The chopped off C becomes CO2
- Now we have a 5-C a-KETOGLUTARATE
36
Reaction 5the second oxidation
- The 5-C a-KETOGLUTARATE is decarboxylated
- The 4-C fragment that is left is called a
Succinyl group CoA ? succinyl CoA - 2 more e- reduce another NAD to NADH
- The second CO2 is released
37
Reaction 6Substrate-level phosphorylation
- The bond between the succinyl group and CoA is
high energy - GDP Pi ? GTP just substitute guanine for
adenine in A TP - GTP? ATP
- Remaining 4-C molecule is SUCCINATE
38
Reaction 7The third oxidation
- Succinate oxidized to fumarate
- Not enough ?G for the NAD reaction so FAD
FOUR e- and TWO H ? FADH2 - FADH2 can contribute e- to ETS
39
Reactions 8 9Regeneration of OXALOACETATE
- Water is added to 4-C FUMARATE forms 4-C MALATE
- Malate oxidized to 4-C OXALOACETATE and 2 e- are
released - NAD the 2 e- ? NADH
- Ready to start again!
40
(No Transcript)
41
Summary So Far
42
ETS or ETCHarvesting e- in stages
- Theres thousands of KJ of energy stored in the
gas tank of a car - If you release it all at once, most is wasted
- Cells are no different
- NADH contains 2 e- H
- If all the energy is released at once, most is
wasted - Better to release in stages? ETS
43
(No Transcript)
44
Following the Electrons
- 2 e- pass along the ETS in the presence of
oxygen - Structure of ETS
- Series of proteins embedded in INNER MEMBRANES OF
MITOCHONDRIA cristae - e- delivered BY NADH to top of chain
- e- captured by oxygen at the bottom, thus E is
released in stages
45
The proteins embedded
- NADH carries its 2 e- to NADH hydrogenase
- Ubiquinone carries e- to the cytochromes
- This complex of cytochromes acts as a proton
pumpdrives protons outside of membrane
46
ETS Proteins continued
- Cytochrome c carries e- to cytochrome oxidase
complex - 4 e- are used to reduce one oxygen molecule
- This oxygen molecule combines with 4 H to form
water
47
(No Transcript)
48
Differences between NADH FADH2
- NADH carries e- to the FIRST position in chain
AND has higher energy e- s - FADH2 caries e- later down the chain and carries
lower energy e- s
49
Building an Electrochemical Gradient
- ETS transports e- along the chain
- E transports H to outer compartmentintermembrane
space - Transport accomplished by proton pumps,
transmembrane proteins - NADH activates 3 pumps
- FADH2 activates 2 pumps
- STILL NO ATP!!!
50
CHEMIOSMOSISmanufacturing ATP
- Concentration of protons H in outer
compartment increases over inner compartment
matrix - H attracted back inward through ATP synthase
- ATP is synthesized when protons diffuse through
them this is chemiosmosis, and makes ATP by
oxidative phosphorlyation. - ATP leaves the mitochondrion by facilitated
diffusion
51
ATP Synthase Animation
- http//www.labaction.com/view_video.php?viewkey75
e45bba028a21ed3804
52
Theoretical ATP Yield
- Since NADH activates 3 pumps, expect 3 ATP 10
NADH 30 ATP - Since FADH2 activates 2 pumps, expect 2 ATP 2
FADH2 4 ATP - NADH transport from glycolysis costs 1 ATP4 were
made, so really costs 2 ATP leaving 2 ATP - GRAND TOTAL 36 ATP expected
53
ACTUAL YIELD
- Must obey the First Law of Thermodynamics so the
net is closer to 30 ATP in eukaryotes - Inner membrane is leaky, so some H sneak in
without producing ATP - Mitochondria use proton gradient for other
purposes - 2.5 ATP/NADH 1.5 ATP/FADH2 is more realistic
54
Regulating ATP production
- If ATP is high, glycolysis, Krebs and fatty acid
breakdown is inhibited. This is an example of
feedback inhibition - When ATP is low, ADP is high which activates
enzymes of carbohydrate catabolism - If NADH levels are high, no decarboxylation of
pyruvate to get Krebs startedNADH inhibits enzyme
55
You cannot live on sugar glucose
alone! --your Mom
- Proteins
- Break into AAs
- Deaminate
- Alanine to pyruvate
- Glutamate to a ketoglutarate
- Aspartate to oxaloacetate
- AAs join the Krebs cycle at different points
56
- FATS
- Degrade into individual fatty acids glycerol
- Oxidized in matrixenzymes attack long fatty acid
chains and remove 2C chunks - Entire chain is converted into acetyl-CoA
- Called Beta oxidation
- Glycerol is converted into pyruvate.
57
Biosynthesis
- When there is an excess of intermediates they can
be used to build necessary molecules. - Lipids can be generated from excess acetyl CoA
- Glycogen is generated from excess pyruvate
- Amino acids are genertated from different stages
of the krebs cycle.
58
Metabolism Summary