Covalent Bonding - PowerPoint PPT Presentation
1 / 22
Title:
Covalent Bonding
Description:
Covalent Bonding. Covalent Bond: a bond where atoms share electrons ... Trigonal Planar: Bent: Pyramidal: Tetrahedral: Trigonal Bypyramidal: Hybridization: when ... – PowerPoint PPT presentation
Number of Views:157
Avg rating:3.0/5.0
Title: Covalent Bonding
1
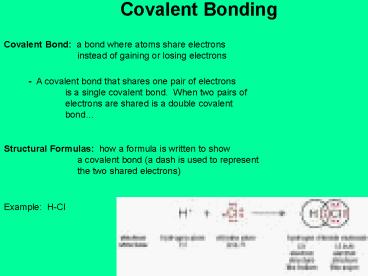
Covalent Bonding
Covalent Bond a bond where atoms share
electrons instead of gaining or losing
electrons
- A covalent bond that shares one pair of
electrons is a single covalent bond. When two
pairs of electrons are shared is a double
covalent bond
Structural Formulas how a formula is written to
show a covalent bond (a dash is used to
represent the two shared electrons)
Example H-Cl
2
- Covalent compounds are called
molecules (subscripts are not necessarily in the
lowest whole number ratio like ionic compounds)
- The octet rule still applies (so each element
still wants to have eight valance electrons)
except for hydrogen.
Unshared Pairs (Lone Pairs) pairs of electrons
that are not involved in a covalent bond
3
We learned to write electron dot notation so that
electrons that share an orbital will be
written on the same side of the elemental symbol.
- When electrons are given a little bit of
energy electrons can move to higher energy
sublevels for bonding purposes
- This is why carbon makes four bonds
4
Double and Triple Covalent Bonds
Double Bonds when two pairs of electrons are
shared between two atoms
Triple Bonds when three pairs of electrons are
shared between two atoms
- Atoms will make double or triple bonds to
meet the requirements of the octet rule
5
Coordinate Covalent Bonds
Typically in a bond, each atom contributes one
electron to each bond.
Coordinate Covalent Bond a covalent bond
where both electrons in the bond are
contributed from one atom.
- Atoms in polyatomic ions are
coordinate covalently bonded.
6
Bond Dissociation Energy the energy it
takes to break a bond.
- The more energy it takes to break a bond the
stronger that bond is.
7
Resonance Structures
Sometimes it is possible to draw the dot
structures for one molecule in more than one
way.
- The molecule does not flip back and forth
between the two structures it is an
average between the two.
- The more resonance structures a molecule has
the more stable that element is.
8
Exceptions to the Octet Rule
When electrons share an orbital the have
opposite spins. If a molecule has only paired
electrons the spins will cancel out and it will
be diamagnetic
Paramagnetic when a molecule has unshared
electrons the spin of the electrons will not
cancel each other out.
- These molecules show a strong attraction in a
magnetic field
9
Other Exceptions to the Octet Rule
Other atoms may expand there octet and take on
10 or 12 electrons.
10
Properties of Covalent Molecules
- nonmetalic
- Could be a solid, liquid, or gas
- Low melting points
- Low solubility
- Poor electrical conductors
11
Bonding Theories
VSEPR Theory
Valence Shell Electron Pair Repulsion Theory
- Electron pairs repel
- Electron pairs are as far away from
other pairs of electrons as possible in the
geometric structure of the molecule
12
Shapes of Molecules
Because each pair of electrons wants to be as far
from another pair as possible, we can predict
the shape of the molecule.
Possible shapes
Linear
Pyramidal
Tetrahedral
Bent
Trigonal Planar
Trigonal Bypyramidal
13
Hybridization when electrons move between
two different orbitals to make hybrid orbitals
14
Polar Bonds
Nonpolar Covalent Bonds the atoms pull the
electrons toward them equally
Example the diatomic molecules
Polar Covalent Bonds two different atoms pull
(share) the electrons unequally
- Polarity all depends on the electronegativity
of each atom has in the molecule or their
attraction for electrons.
15
Look at page 405 in your book.
- This is a table of electronegativities
- To determine if the bond between two atoms is
polar subtract the electronegativities of the
two atoms involved in the bond.
16
Polar Molecules molecules will become polar if
one end of the molecule is more
electronegative then the other end
- All of the electrons will be pulled to the
atom that is most electronegative
- This will make the molecule negatively
charged on one end and positively charged at
the other end.
17
Rules of Thumb for Polar Molecules
- If a bond in a two atom molecule is polar then
the molecule is polar.
- If the molecule is more than two atoms, then
you must also look at the shape to determine if
the molecule is polar.
- Symmetric shapes, where the polar bonds
cancel each other out lead to nonpolar molecules.
18
Attractions Between Molecules
Molecules have attractions towards other
molecules.
- These attractions are not as strong as bonds
Van der Waals Forces
1. Dispersion Forces the attractions that
occur with nonpolar molecules
- This happens because the nucleus of each atom
is attracted to the electrons of other molecules
19
2. Dipole Attractions the attractions
between polar molecules.
- The slightly positive end of one molecule is
attracted to the slightly negative end of
another molecule.
20
Hydrogen Bonds (a type of dipole
attraction) Attractive forces that occur
between the hydrogen of one molecule and the
highly electonegative element of
another molecule when hydrogen is covalently
bonded to a highly electronegative element.
21
This is why water has such an unexpected
high boiling point and why it gets larger when
it freezes.
- To boil water you have to add more energy
to break all of the hydrogen bonds.
- When water freezes the molecules of water are
all organized with hydrogen bonds. This makes
water expand when freezing.
Hydrogen bonding is the strongest of the
intermolecular forces.
22
Although most molecular compounds are
unstable there is an exception
Network Solids (Network Crystals) solids in
which all the atoms are covalently bonded to
eachother.
- These are very hard substances such as
diamonds