Chemical Arithmetic - PowerPoint PPT Presentation
1 / 27
Title:
Chemical Arithmetic
Description:
... with an internal pressure of 720. torr at 20 C is thrown into an incinerator operating at 750 C. ... 28.50 mL of H2(g), measured at 26 C and 758 Torr? ... – PowerPoint PPT presentation
Number of Views:388
Avg rating:3.0/5.0
Title: Chemical Arithmetic
1
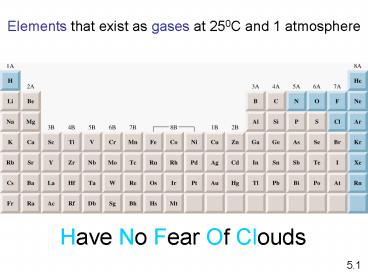
Elements that exist as gases at 250C and 1
atmosphere
Have No Fear Of Clouds
5.1
2
5.1
3
Microscopic View of Molecular Solids, Liquids and
Gases
- Not looking at ionic substances, or metals or
network covalent substances or atomic substances - Gaseous state - molecules are in constant rapid
motion, are widely separated, are not attracted
to each other and when they collide they bounce
off each other - Liquid state - molecules are moving slowly, are
close to each other, have attractions to each
other that are constantly forming and breaking - Solid state - molecules are close together, have
strong attractions for each other, are locked
into position and only have vibrational motion
4
Transitions between states
- Addition of energy to the molecules, usually as
heat (increase in temperature), causes transition
to more dispersed state (state with more kinetic
energy motion) - Melting ice, boiling water
- Removal of energy from the molecules through
cooling causes transition to more organized state
(state with more potential energy attractions) - Freezing water, condensing steam
5
Gases - Kinetic Molecular Theory
- The molecules do not attract or repel each other.
- Each molecule behaves as an independent particle.
- The average distance between the molecules is
very large in comparison to the size of a
molecule. - Most of the container is empty space.
- BUT gases can be compressed
- The average speed of the molecules increases as
the temperature increases. - Gas molecules are in constant, random motion,
colliding with each other and the walls of the
container.
6
Pressure macro and microscopic
- Pressure - microscopic level (from Kinetic
Molecular Theory) - number and power of collisions of the molecules
with the walls of the container (including where
the gauge is located) per second - small gas molecules move around faster and hit
the sides of the container more often but with
low power larger gas molecules move slower, hit
less frequently but hit with more power - Pressure - macroscopic level (observable)
- Force exerted per unit area
- Pressure of different shoes
- Force felt at different depths
- In water
- In air
7
Pressure
(force mass x acceleration)
Units of Pressure
1 pascal (Pa) 1 N/m2 1 atm 760 mmHg 760
torr 1 atm 101,325 Pa
5.2
8
10 miles
0.2 atm
4 miles
0.5 atm
Sea level
1 atm
5.2
9
Figure 5.4
5.2
10
As P (h) increases
V decreases
5.3
11
Pressure - mathematical
- Mathematical what can be quantified and
compared - Pressure constant x ½mv2
- Units
- lbs/in2 (everyday units) 1 atmosphere (atm)
760 mm Hg (scientific units) Pascals - For a fixed amount of any gas under the same
conditions, the pressure is the same Ideal Gas
Law - the differences in power and speed exactly cancel
each other - 20 hits x 2 push units/hit 40 push units
- 10 hits x 4 push units/hit 40 push units
- 5 hits x 8 push units/hit 40 push units
12
Common Sense Observations about Gases
- Pressure gauges on hard wall containers
- the more gas you put in, the higher the pressure
P (pressure)
n (moles)
- Pressure cookers
- the higher the temperature, the higher the
pressure
P (pressure)
T (temperature)
- Pistons or Syringes
- the pressure increases as the piston is pushed in
P (pressure)
V (volume)
13
Absolute Ideal Gas Law
PV nRT
- P Pressure (atmospheres)
- V Volume (Liters)
- n number of moles (moles)
- R gas constant 0.082 L-atm/mole-K
- T Temperature in K (oC 273)
- Sense of the equation
- fits common sense observations
- For equation to work correct units have to be used
14
Using the Absolute Ideal Gas Law
- Start with the memorized equation
PV nRT
- Rearrange to solve for unknown component
- Change each of the quantities to the correct
units (if needed) - Insert values and calculate (convert back to oC
if required) - Does your answer make sense!!!!
15
Generalized Relative Ideal Gas Law
- Rearrange ideal gas law to comparative form
PV nRT
- Since any set of conditions for a certain gas is
equal to a constant we can compare two different
conditions for any gas
- For the relative law, units are not as important
as long as they are consistent BUT temperature
must always be in K
16
Relative Gas Laws
- The generalized relative gas law can be
simplified depending on what variables are
present in the problem - Consider a problem that involves only pressure
and volume
- Start with the generalized law then cross out
variables that are not in the problem - Rearrange the variables to solve for the unknown
component - Add the values that are known (convert T to units
of K if needed) - Do math (convert back to oC if you have to)
- Does your answer make sense!!!!
17
Solving gas law problems
- Figuring out which equation to use
- Relative cues
- Look for two volumes, or two pressures or two
temperatures or two number of moles - Look for change or new or will be
- Absolute cues
- Only one of each variable given
- R often given
- Often if g of gas given (need to get to moles)
- Reducing/rearranging equation to get unknown
- Changing all variables to appropriate units that
can be used in equations - Always have to use K
- Absolute always have to use L, atm, moles
- Relative can use different volume and pressure
units as long as consistent
18
Example 1 of using strategy
- Problem What is the volume, in milliliters,
occupied by 89.2 g CO2 (g) at 37 ºC and 737 mmHg? - Solution
- Cues
- g of CO2 ?absolute
- Only one of each variable ? absolute
- Equation need volume ? V nRT/P
- Conversions
- 89.2 g of CO2 x 1 mole CO2/44.0 g CO2 2.03
moles - 37 273 310 K
- 737/760 0.970 atm
- R 0.082 L-atm/mole-K
- Substitute and solve V 2.03x0.082x310/.970
53.1 L
19
Example 2 of using strategy
- Problem A sample of gas has a volume of 4.25 L
at 25.6 ºC and 748 mmHg. What will be the volume
of this gas at 26.8 ºC and 742 mmHg? Solution - Cues
- Will be ? relative
- Two temperature two pressures
- Reduce equation (moles constant) ? P1V1/n1T1
P2V2/n2T2 - Conversions
- T1 25.6 273 298.6 K T2 26.8 273
299.8 K - Can use pressure values as given and L value
- Substitute and solve P1V1/T1 P2V2/T2
- (748)(4.25)/298.6 (742)V2/299.8
- ALGEBRA!!!!
- V2 4.30 L
20
Some more problems 1
- A light bulb with an internal pressure of 720.
torr at 20ºC is thrown into an incinerator
operating at 750ºC. What internal pressure must
the light bulb be able to withstand if it does
not break? - Relative P and T only make sure convert T to K
- A 12.8-L cylinder contains 35.8 g O2 at 46 ºC.
What is the pressure of this gas, in atmospheres? - Absolute convert g O2 to moles convert T to K
21
Some more problems 2
- A 72.8-L constant-volume cylinder containing 1.85
mol He is heated until the pressure reaches 3.50
atm. What is the final temperature in degrees
Celsius? - Absolute only one of each OK to go ? get T
- BUT ? convert back to oC
- If a 1500.-mL sample of air at 22 ºC is cooled
enough to cause the volume to decrease to 750. mL
at constant pressure, what is the final Celsius
temperature required? What was the temperature
change? - Relative Two volumes V (use mL) and T only
convert T to K - Get K answer ? convert back to oC also get
temperature change
22
Gas Stoichiometry
- Stoichiometry with gases obeys the same rules as
stoichiometry with g moles molarity - Usually relationships in equations in which gas
is consumed or produced - Two general problems
- Information about gases known and information
about other components in equation sought - Information about other components known and
information about gases in equation sought - Can expand switching yard!!
23
Gas Stoichiometry
PV nRT
24
How many mg of magnesium metal must react with
excess HCl(aq) to produce 28.50 mL of H2(g),
measured at 26 ºC and 758 Torr? Mg (s) 2 HCl
(aq) ? H2 (g) MgCl2 (aq)
PV/RT? moles of H2 ? moles of Mg ? g of Mg
n 1.16 x10-3 moles H2
1.16 x10-3 moles H2
1 mole Mg 1 mole H2
1.16 x10-3 moles Mg
24.3 g Mg 1 mole Mg
0.0282 g Mg
28.2 mg Mg
0.0282 g Mg
25
What volume of CO2 (g) is produced at a
temperature of 20. ºC and a pressure of 1.00 atm
by the fermentation of 500. g of glucose?
C6H12O6 ? 2 C2H5OH 2 CO2 (g)
g of glucose ?moles of glucose ? moles of CO2 ?V
of CO2 ( PV/RT)
V 134 L
5.56 mole CO2
2 mole CO2 1 mole glucose
2.78 mole glucose
1 mole glucose 180 g glucose
500 g glucose
0.0282 g Mg
26
A 3.57-g sample of a KCl-KClO3 mixture is
decomposed by heating and produces 119 mL O2 (g),
measured at 22.4 ºC and 738 mmHg. What is the
mass percent of KClO3 in the mixture? 2 KClO3
(s) ? 2 KCl(s) 3 O2 (g)
Moles of O2 ? moles of KClO3 ? g of KClO3 ?
KClO3 of 3.57g
n 4.77 x10-3 moles O2
4.77 x 10-3 mole O2
2 mole KClO3 3 mole O2
3.18 x 10-3 mole KClO3
122.6 g KClO3 1 mole KClO3
0.390 g/3.57 g x 100
10.9
0.390 g KClO3
0.0282 g Mg
27
Complex gas stoichiometry
- How many liters of SO3(g) can be produced by the
reaction of 1.15 L SO2(g) and 0.65 L O2(g) if all
three gases are measured at the same temperature
and pressure? - 2 SO2(g) O2(g) ? 2 SO3(g)
- Since R is a constant the term RT/P is a constant
since we are not changing temperature and
pressure - We can therefore compare volumes in balanced
equations just like we can compare moles - This only applies if T and P are constant
- This is a limiting reagent problem
- SO2 O2
- HAVE 1.15 L 0.65 L
- NEED 2(0.65)L ½ (1.15)L
- 1.30 L 0.575 L
- SO2 is the limiting reagent since we need 1.30 L
to react with O2 but only have 1.15 L. Therefore
we will form the same amount of SO3 gas - 1.15 L