Chemical reactions qualitative and quantitative information - PowerPoint PPT Presentation
1 / 12
Title:
Chemical reactions qualitative and quantitative information
Description:
Qualitatively : stoichiometry of the reaction process : number of molecules of ... As the basic law of Nature is the law of conservation of matter (and energy) ... – PowerPoint PPT presentation
Number of Views:99
Avg rating:3.0/5.0
Title: Chemical reactions qualitative and quantitative information
1
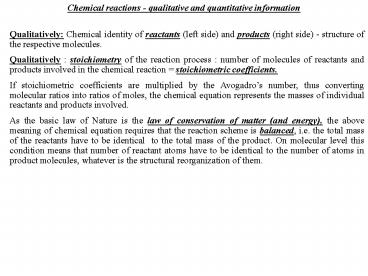
Chemical reactions - qualitative and quantitative
information
Qualitatively Chemical identity of reactants
(left side) and products (right side) - structure
of the respective molecules. Qualitatively
stoichiometry of the reaction process number of
molecules of reactants and products involved in
the chemical reaction stoichiometric
coefficients. If stoichiometric coefficients are
multiplied by the Avogadros number, thus
converting molecular ratios into ratios of moles,
the chemical equation represents the masses of
individual reactants and products involved. As
the basic law of Nature is the law of
conservation of matter (and energy), the above
meaning of chemical equation requires that the
reaction scheme is balanced, i.e. the total mass
of the reactants have to be identical to the
total mass of the product. On molecular level
this condition means that number of reactant
atoms have to be identical to the number of atoms
in product molecules, whatever is the structural
reorganization of them.
2
Chemical equation
Additional qualitative information
Additional quantitative information
How many how many How many how
many molecules of A molecules of
B molecules of M molecules of N
Multiply by Avogadro number NA
How many how many How many how many
moles of A moles of B moles of M moles
of N
3
Balancing chemical equation
4
??
Balancing this chemical equation
5
Reactants
Products
6
C7H16 (l) O2 CO2 H2O
7
(No Transcript)
8
Balanced chemical equation
9
Reactants Products
10
Checking the mass balance
Left side (reactants)
Right side (products)
3x26 H 3x13 S 3x412 O 2x12 Al 2x36 Cl
6x16 H 3x13 S 3x412 O 2x12 Al 6x16 Cl
Chemical reaction is balanced !
11
Multiply both sides by integer number
10 x
10 x
12
Instead by 10, multiply both sides of equation by
Avogadro number
Implicit information chemical equation can be
read in terms of molecules or moles (of
molecules) of reactants and products