Steroid hormones - PowerPoint PPT Presentation
1 / 23
Title:
Steroid hormones
Description:
The most numerous and most complex monoxygenation ... (rhodopsin in rods, iodopsin in cones) 23:6. Rhodopsin = an opsin protein bonded to a molecule of retinal ... – PowerPoint PPT presentation
Number of Views:138
Avg rating:3.0/5.0
Title: Steroid hormones
1
Steroid hormones
231
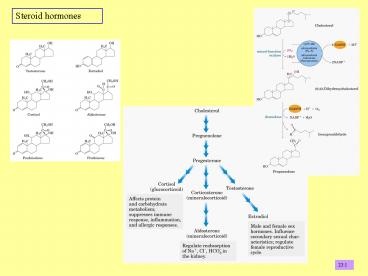
2
Synthesis of steroid hormones
232
3
Cytochrome P-450
- The most numerous and most complex monoxygenation
reactions are those using cytochrome P-450 - Cytochrome P-450s are generally found in the ER
(instead of mitochondria) - Cytochrome P-450 is a family of several hundred
very similar proteins, each with a different
substrate specificity. - They catalyze hydroxylation reactions in which an
organic substrate RH is hydroxylated to R-OH,
incorporating one oxygen atom of O2 - The other oxygen atom is reduced to H2O by
reducing equivalents furnished by NADH or NADPH
but usually passed to P-450 by an iron-sulfur
protein
Cytochrome P-450 is also important in the
hydroxylation of xenobiotics (substances that are
foreign to the body) Example hydroxylation of
barbituates makes them more soluble and allows
their excretion in the urine
Note however that sometimes the hydroxylation of
a compound can convert it into a toxic substance
233
4
234
5
Vitamin A
plays an important role in vision also involved
in nervous system development can be consumed
directly in the diet or biosynthesized from
?-carotene in the diet
There are three forms of Vitamin A retinol,
retinal, retinoic acid
a specific transport protein, retinol binding
protein (RBP) picks up vitamin A from the liver
(where it is stored) and transports it to the
blood stream cell that use vitamin A have
specific receptors for its different forms
Vitamin A in differentiation vitamin A in
epithelial cells is involved in differentiation
in ways not well understood yet, but deficiencies
of vitamin A can lead to decreased cell division
Beta-carotene as an antioxidant ?-carotene
functions in the body as a vitamin A precursor,
but on its own acts as an anti-oxidant
235
6
Vitamin A in vision
light passes through the cornea of the eye and
strikes the cells of the retina where pigment
molecules inside the cells of the retina absorb
light (rhodopsin in rods, iodopsin in cones)
236
7
Vitamin A in vision
Rhodopsin an opsin protein bonded to a molecule
of retinal the retinal molecule absorbs a photon
of light and responds by changing shape (shifting
from a cis to a trans configuration) In the
process, the retinal also changes color, becoming
bleached retinal is released from opsin, which
changes shape and sends a signal to a nerve
cell some retinal is converted back to its
original form and rejoined with opsin, but some
is oxidized to retinoic acid - a biochemical dead
end for vision
This retinal that is lost during visual activity
needs to be replenished (from vitamin A stores in
the liver)
237
8
Vitamin A and night blindness
- at night, light entering the eye is of low
intensity and can be received only by the rod
cells (so at night, a person can normally discern
only the presence of light and not its color) - 7 million cone cells to 100 million rods cells in
a retina - each cell containing 30 million molecules of
retinal - A lot of retinal can be destroyed at night so if
vitamin A stores are marginal, the use of the
eyes at night can lead to vitamin A deficiency
(night blindness - an early symptom)
238
9
Vitamin E (?-tocopherol)
tocopherol is greek to bring forth offspring
originally identified as an agent that
prevented sterility in rats while ?-tocopherol
is the most biologically active vitamin E
compound, there are also beta, gamma and delta
tocopherols a fat soluble antioxidant, that is
especially effective in preventing the oxidation
of polyunsaturated fatty
acids widespread in food, especially vegetable
oils (animal fats contain little or no vitamin
E) easily destroyed by heat and
oxidation tocopherols occur in two forms, D and
L and D is the most active vitamin E deficiency
is rare and usually associated with diseases of
fat malabsorption when blood concentration of
vitamin E falls below a certain critical level,
red blood cells tend to break open - probably due
to the oxidation of polyunsaturated fatty acids
in their membranes
239
10
Vitamin K
- (K stands for the Danish work koagulation)
- originally identified as a lipid-soluble
substance involved in blood coagulation - vitamin K1 (phylloquinone) is found in plants
- vitamin K2 (menaquinone) is found in animals and
bacteria) - In animals, vitamin K2 is essential for the
carboxylation of glutamate residues in certain
proteins (yielding ?-carboxyglutamate) - This modification allows proteins in the blood
clotting cascade to bind calcium - Vitamin K deficiencies can occur whenever
absorption of fat is impaired - Intestinal bacterial synthesize vitamin K that
the body can absorb - (but more needs to be obtained from the diet)
- High doses of vitamin K can reduce the
effectiveness of anticoagulants
2310
11
Other terpenes
terpenes is a generic term for all compounds
biosynthesized from isoprene precursors
2311
12
2312
13
Eicosanoids (Prostaglandins, thromboxanes,
leukotrienes)
- Substances that act only on cells near the point
of synthesis (instead of being transported in the
blood to act on cells in other tissues or organs) - Involved in
- Inflammation
- Fever
- Pain
- Formation of blood clots
- Regulation of blood pressure
- Gastric acid secretion
- Reproduction
- Wake/sleep cycle
- All eicosanoids are derived from the 20-carbon
polyunsaturated fatty acid - arachidonic acid 204(D5,8,11,14)
- Very difficult to study because of
- Extremely low levels in tissues
- Extremely rapid metabolic turnover
2313
14
mammalian cells also contain ?5 and ?6
desaturases mammalian cells cannot introduce
additional double bonds into the fatty acid chain
beyond ?9
When linoleic acid and linolenic acid are
ingested by animals, they are then substrates for
further elongation and desaturation reactions
Linoleic acid
note that most of the arachidonyl-CoA produced in
this pathway is used for phospholipid synthesis
and that arachidonic acid used for eicosanoid
biosynthesis is derived from hydrolysis of these
phospholipids
Linolenic acid
2314
15
2315
16
The synthesis of PGH2
A cycloxygenase activity two molecules of O2
are introduced Arachidonate is converted to
PGG2 (prostaglandin G2)
PGH synthase is a bifunctional enzyme
B peroxidase activity a two electron reduction
of the peroxide PGG2 is converted to PGH2
Cycloxygenase activity of PGH synthase is
inhibited by aspirin and NSAIDS (non-steroidal
anti-inflammatory drugs)
2316
17
Structure of Cox1
- Two identical monomers each have three domains
- A membrane anchor consisting of four amphipathic
helices - A domain that resembles EGF
- Catalytic domain which contains the cylooxygenase
and peroxidase activities
Catalytic domain also contains a hydrophobic
channel that binds substrate
Red heme group Med blue tyr385 Dark blue
arg120 Green his 388 Yellow ser530 Orange
flurbiprofin Blocks access to the substrate
binding site
2317
18
Aspirin inhibits binding of substrate to PGHS
(COX) by acetylating serine 530
Aspirin acetylates both COX1 and COX2
COX1 and COX2 have similar amino acid sequences
and similar reaction mechanisms at their
catalytic centers
However COX1 and COX2 have different
functions (they are responsible for the ultimate
synthesis of different prostaglandins) COX-1 is
responsible for the synthesis of the
prostaglandins that regulate the secretion of
gastic mucin COX-2 for the prostaglandins that
mediate inflammation, pain and fever
Aspirin inhibits COX1 and COX2 equally Therefore
a dose of aspirin sufficient to reduce pain can
also cause stomach irritation (including bleeding
of the stomach)
Research is aimed at COX-2 specific inhibitors
2318
19
The mechanism of blocking access of substrate to
the binding site varies among NSAIDS
Acetominophen and ibuprofen bind non-covalently
to the active site
Ibuprofen forms a hydrogen bond with Arginine 120
Ibuprofen acts more specifically on COX2, but
is a less effective inhibitor than aspirin
COX 1 has an isoleucine at postion523 (COX 2 has
a valine (smaller by a single CH2) Presence of
the less bulky valine allows entry of COX2
specific inhibitors
Celebrex introduced in 1998 binds 400 fold more
tightly to COX2 than to COX1
Glucocorticoids (cortisol) inhibit phospholipase
A2
2319
20
thromboxanes
Thromboxane synthase is present in blood
platelets it converts PGH2 into thromboxane
A2 Thromboxanes induce constricton of blood
vessels and platelet aggregation early steps in
blood clotting
How do regularly taken low doses of aspirin
reduce the probability of heart attacks and
strokes?
The key is the fact that platelets are
enucleated Remember that the acetylation of
serine 530 by aspirin is irreversible Without a
nucleus, platelets cannot generate any more PGH
synthase While PGH synthase in all cells is
initially inhibited by aspirin, cells with a
nucleus can make more PGH synthase but platelets
cannot so the synthesis of thromboxane A2 is
inhibited
2320
21
Polyunsaturated fatty acids and risk factors for
heart disease
Epidemiological studies have demonstrated that
the ratio of polyunsaturated fats (PUFAs) to
saturated fats (SFAs) in the diet affect serum
cholesterol levels Most recently it has been
determined that different types of
polyunsaturated fatty acids have different
effects on lipid metabolism and on other risk
factors for heart disease
Linoleic (w-6) Linolenic (w-3)
There are two families of polyunsaturated
essential fatty acids
Recent clinical studies have shown that w-6
PUFAs (plants and vegetable oils) decrease serum
cholesterol levels (some lowering of TAG
levels) w-3 PUFAs (eicosapentaenoic acids in
certain ocean fish and fish oils) Decrease serum
TAG levels (some lowering of cholesterol
levels) Biochemical mechanisms behind these
effects remains to be elucidated
Omega-3 PUFAs also decrease platelet aggregation
2321
Greenland eskimos
22
leukotrienes
Synthesized by mixed function oxidases that are
present in heart, brain, lung and spleen The
pathway of synthesis of leukotrienes is not
inhibited by NSAIDS Mediators of immune mediated
inflammatory reactions of anaphylaxis Cause
constriction of bronchial smooth muscle and
anti-leukotriene agents are being used in
treatment of allergen and exercise-induced asthma
2322
23
Some Background on Eicosanoids and aspirin
1763 first description by western science of the
medicinal properties of compounds known as
salycilates It was noted that the bark of the
willow tree Salix alba was effective against
fevers, aches and pains 1830s German scientist
purified the active components from willow and
from another plant rich in salicylates Spiraea
ulmaria Early 1900s aspirin (a for acetyl
spir for Spiraea ) was being widely
used 1930s prostaglandins were
discovered 1960s synthetic pathways
elucidated 1971 discovery that aspirin
inhibited one of the enzymes in prostaglandin
biosynthesis 1970s thromboxanes and
leukotrienes discovered 1998 Celebrex
2323