Process Classification - PowerPoint PPT Presentation
1 / 13
Title:
Process Classification
Description:
Batch no material is transferred into or out of the system over ... Reflux Condenser. Bottoms Reboiler. Separator Heat. Exchanger. Splitter Heat Exchanger ... – PowerPoint PPT presentation
Number of Views:172
Avg rating:3.0/5.0
Title: Process Classification
1
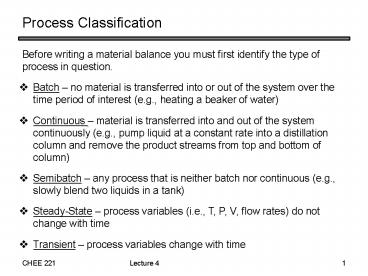
Process Classification
- Before writing a material balance you must first
identify the type of process in question.
- Batch no material is transferred into or out of
the system over the time period of interest
(e.g., heating a beaker of water) - Continuous material is transferred into and out
of the system continuously (e.g., pump liquid at
a constant rate into a distillation column and
remove the product streams from top and bottom of
column) - Semibatch any process that is neither batch nor
continuous (e.g., slowly blend two liquids in a
tank) - Steady-State process variables (i.e., T, P, V,
flow rates) do not change with time - Transient process variables change with time
2
Example
- Classify the following processes as batch,
continuous, or semibatch, and transient or
steady-state. - A balloon is filled with air at a steady rate
of 2 g/min. - A bottle of milk is taken from the refrigerator
and left on the kitchen table. - Water is boiled in an open flask.
- Carbon monoxide and steam are fed into a
tubular reactor at a steady-rate and react to
form carbon dioxide and hydrogen. Products and
unused reactants are withdrawn at the other end.
The reactor contains air when the process is
started up. The temperature of the reactor is
also constant, and the composition and flow rate
of the entering reactant stream are also
independent of time. Classify the process (a)
initially and (b) after a long period of time
has elapsed.
3
Material (Mass) Balances
- A material balance is simply an accounting of
material. For a given system, a material balance
can be written in terms of the following
conserved quantities - System a region of space defined by a real or
imaginary closed envelop (envelopsystem
boundary) - may be a single process unit, collection of
process units or an entire process
- Total mass (or moles)
- Mass (or moles) of a chemical compound
- Mass (or moles) of an atomic species
To apply a material balance, you need to define
the system and the quantities of interest.
4
General Balance Equation
Accumulation In Out Generation
Consumption
system boundary
Input streams to system
output streams from system
-
5
Integral Versus Differential Balances
- Integral Balances written in terms of the
amounts of a specified quantity over a period of
time - E.g. At the start of the year my bank balance
was 4328. Now it is 2555. - Accumulation (final output initial input)
- Typically applied to batch processes
- Differential Balances written in terms of rates
of change of the specified quantity with respect
to time - E.g. I get paid 10.50/hr
- Accumulation is a differential term
- Typically applied to continuous steady-state
processes - Used extensively in this course
6
Example
- A country has a population of 10 million people
in 1900 AD. Over the period from 1900 to 2000, 6
million people immigrated into the country, 2
million people emigrated from the country, 5
million people were born in the country and 3
million people died in the country. What is the
population of the country in the year 2000 AD?
7
Material Balance Simplifications
- The following rules may be used to simplify the
material balance equation
Accumulation In Out Generation
Consumption
- If the system is at steady-state, set
accumulation 0 - If the balanced quantity is total mass, set
generation 0 and consumption 0 (law of
conservation of mass) - If the balanced substance is a nonreactive
species, (neither a reactant nor a product), set
generation 0 and consumption 0
? In Out Generation Consumption 0
? Accumulation In Out
? Accumulation In Out
8
Process Unit Basic Functions
- Splitter divides a single input into two or
more outputs of the same composition (no
reaction) - Mixer combines two or more inputs (usually of
different compositions) into a single output) (no
reaction) - Separator separates a single input into two or
more outputs of different composition (no
reaction)
splitter
mixer
separator
9
Process Unit Basic Functions
- Reactor carries out a chemical reaction that
converts atomic or molecular species in the input
to different atomic or molecular species in the
output - Heat exchanger transfers heat from one input to
a second input (no reaction) - Pump changes the pressure of an input to that
of the corresponding output (no reaction)
Actual process units usually combine these
different functions into a single piece of
hardware, and are given different names.
10
Steam Boiler
Steam Boiler
Heat Exchanger Reactor
11
Distillation
Column
Separator
Splitter Heat Exchanger
Reflux Condenser
Separator Heat Exchanger
Bottoms Reboiler
12
Distillation Inside the Column
- Internal trays (or packing) are used to
enhance component separations - Each tray accomplishes a fraction of the
separation task by transferring the more
volatile species to the gas phase and the less
volatile species to the liquid phase
(Thermodynamics!!) - Can perform material and energy balances on
- an individual tray
- the column, bottoms reboiler, or top condenser
- the entire system
white vapour black liquid
13
Problems Involving Material Balances
- Procedures will be outlined for solving single
unit processes - No reaction (consumption generation 0)
- Continuous steady-state (accumulation 0)
- These procedures will form the foundation for
more complex problems involving multiple units
and processes with reaction - Develop good problem solving habits now!!