Phosphoryl Transfer - PowerPoint PPT Presentation
1 / 18
Title:
Phosphoryl Transfer
Description:
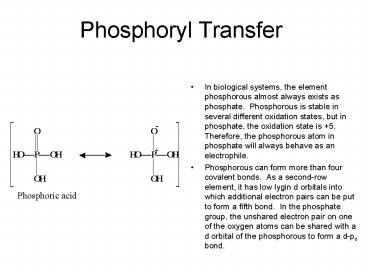
Phosphoryl Transfer. In biological systems, the element phosphorous almost always ... the phosphorous atom in phosphate will always behave as an electrophile. ... – PowerPoint PPT presentation
Number of Views:204
Avg rating:3.0/5.0
Title: Phosphoryl Transfer
1
Phosphoryl Transfer
- In biological systems, the element phosphorous
almost always exists as phosphate. Phosphorous
is stable in several different oxidation states,
but in phosphate, the oxidation state is 5.
Therefore, the phosphorous atom in phosphate will
always behave as an electrophile. - Phosphorous can form more than four covalent
bonds. As a second-row element, it has low lygin
d orbitals into which additional electron pairs
can be put to form a fifth bond. In the
phosphate group, the unshared electron pair on
one of the oxygen atoms can be shared with a d
orbital of the phosphorous to form a d-pp bond.
2
Examples of Phosphoryl Groups in Biochemistry
3
Small Phosphoryl-Containing Molecules
4
Phosphoryl Amino Acids
5
Classes of Phosphoryl Transfer
In kinases, X is almost always ADP. However, GDP
is known to substitute i some cases.
6
Kinases
- Kinases are phosphotransferases that catalyze the
transfer of a phosphoryl group to an acceptor
molecule. Most often, the phosphoryl group comes
from the terminal (gamma) position of adenosine
triphosphate. - There is high negative charge associated with the
triphosphate group of ATP, which shields each
phosphorus against reaction with incoming
nucleophiles. This property makes ATP
kinetically stable int he cell, although
thermodynamically, its hydrolysis is favorable.
In enzyme catalysis, these charges are typically
neutralized in order to facilitate nucleophilic
attack. - Coordination with metal ions. Most often
magnesium. In the cell, ATP is frequently found
associated with magnesium, and the true substrate
is MgATP. - Ion pairing with positively charged amino acids
such as the guanidinium of arginine, or the
lysine ammonium group. - From the structure of ATP, chemical precedent
would indicate that the g-bond would be cleaved
via a dissociative transition state, while the a
and b-bonds would be cleaved via associative
transition states.
7
Adenylate Kinase
- The transfer of phosphoryl groups between
different nucleotides, as well as other small
molecules is important for utilizing and
replenishing the cellular pool of energy-rich
phosphate compounds. Adenylate kinase is a
classic example of enzymes in this class. - Adenylate kinase was formerly known as myokinase
because it is found in high concentrations in
muscle tissue. - The adenylate kinase reaction is isoenergetic. A
phosphoanhydride is cleaved and formed on both
sides of the equation. - Adenylate kinase displays sequential kinetics, in
which both substrates must be bound before any
product is released. - This is distinguished from what is termed
ping-pong kinetics, in which one reactant
modifies the enzyme, and then a second reactant
interacts with the modification.
8
Bi-substrate Enzyme Kinetics
Sequential 1. ordered 2. random
Ping-pong
9
Equations for Bi-substrate Kinetics
B
1/v
VmaxAB
v
KaB KbA AB
1/A
B
VmaxAB
1/v
v
AB KaB KbA KaKb
1/A
10
Sequential Kinetics
- Sequential kinetics can be distinguished from
ping-pong kinetics by initial rate studies. - In practice, measure initial rates as a function
of the concentration of one substrate while
holding the concentration of the second constant.
Next, vary the concentration of the second
substrate and repeat. - Lineweaver-Burk (double-reciprocal) analysis
should yield a family of lines that intersect at
the left of the y-axis of the graph. - Within the realm of sequential reactions lies
ordered sequential and random sequential at the
extreme ends. The equations for the two are
identical therefore, simple initial rate studies
cannot differentiate between the two. - In ordered sequential reactions, one substrate is
obligated to bind to the enzyme before a second
substrate. In random sequential mechanisms there
is no preference. In practice, there is usually
some degree of order in binding.
11
Ordered- vs. Random- Sequential
12
Adenylate Kinase Kinetic Pathway
Adenylate kinase displays a random ordered
kinetic mechanism. In this case, the two
substrates are bound randomly, and are in
equilibrium with the ternary complex
(EMgATPAMP). As in our derivation, this
necessitates that the off rate for each of the
substrates is less than the forward rate constant
for the chemical step. This allows us to replace
Km with Ks. However, it would not be incorrect
to use Km values. Below is typical shorthand
notation for kinetic schemes.
13
Nucleoside Diphosphate Kinase
- Nucleoside diphosphate kinase (NDP Kinase)
catalyzes the transfer of the terminal phosphoryl
group of ATP to a nucleoside diphosphate. - NDP Kinase displays a steady state kinetic
pattern that is distinctly different from that of
adenylate kinase. If one substrate is varied
while the other is fixed at several different
concentrations, a family of parallel lines is
obtained by Lineweaver-Burk analysis. This is
reminiscent of a Ping-Pong reaction.
14
Economy in the Evolution of Binding Sites
- Since adenylate kinase and nucleoside diphosphate
kinase catalyze very similar reactions, why dont
they proceed by similar mechanisms? - NDP kinase catalyzes a symmetrical reaction,
whereas adenylate kinase does not. For NDP
kinase, the product of the ping (MgADP) is
similar in structure to the substrate for the
pong (MgGDP). The only difference involves the
purine rings of each nucleotide. - By using a ping-pong reaction, the enzyme can use
just one binding site for the phsphoryl transfer.
15
UDP-Glucose Pyrophosphorylase
This is a special type of sequential mechanism in
which MgUTP must bind firs, before
glucose-1-phosphate. There is no degree of
randomness. Ordered binding also implies ordered
product release.
16
UDP-Glucose Pyrophosphorylase
- Steady State kinetic equation is similar to
adenylate kinase. Therefore Lineweaver-Burk
plots cannot distinguish the two forms of
sequential reactions. Must do product inhibition
studies. - Stereochemistry indicates inversion however,
incubation of the enzyme with radiolabeled UTP,
followed by gel-filtration shows a radiolabeled
intermediate. Be careful! This is because UTP
or UDP-glucose binds very tightly to the enzyme.
In fact, the enzyme is isolated with UTP and
UDP-glucose tightly bound, and will catalyze an
exchange reaction, which is characteristic of
Ping-pong reactions.
17
Ping-Pong Reaction
18
Galactose-1-P Uridylytransferase