Dehydrohalogenation PowerPoint PPT Presentations
All Time
Recommended
Facts (1) Dehydrohalogenation of alkyl halides exhibits ... rate: RI RBr RCl RF. implies that carbon-halogen bond breaks in the rate-determining step ...
| PowerPoint PPT presentation | free to download
... Stereoelectronic effect H that is removed by base must be anti periplanar to Br No anti periplanar H atoms in trans stereoisomer; ...
| PowerPoint PPT presentation | free to download
Dehydration of alcohols Dehydrohalogenation of haloalkanes REACTIVITY OF ALKENES More reactive than alkanes because: ...
| PowerPoint PPT presentation | free to view
dehydrogenation. dehydration. dehydrohalogenation. industrial ... Acid-Catalyzed Dehydration of Benzylic Alcohols. KHSO4. heat (80-82%) CH2. CH. CHCH3. OH ...
| PowerPoint PPT presentation | free to download
nomenclature - (chapt 5), structure, classification acidity of terminal acetylenes - (chapt 4) alkylation prep - dehydrohalogenation (-2HX) or double -elimination
| PowerPoint PPT presentation | free to download
New Way Chemistry for Hong Kong A-Level Book 3A. New Way Chemistry for Hong ... g. In dehydrohalogenation of bromoethane, ethene and hydrogen bromide are formed ...
| PowerPoint PPT presentation | free to view
ORGANIC CHEMISTRY 171 Section 201 * I. Preparation 2. Dehydrohalogenation Example: alcoholic KOH EtO- (ethoxide ion) in EtOH (ethanol) Press * Hofmann Product ...
| PowerPoint PPT presentation | free to view
Download free PDF Sample@ https://bit.ly/2WD9lCq #ChemicalsAndMaterials #Chemicals #MarketAnalysis Potassium tert-Butoxide is used as a strong non-nucleophilic base in organic chemistry. It plays an active role in dehydrohalogenation reactions. It is also useful for greener amidation of esters. It serves as an intermediate in Mizoroki-Heck-type reactions. Furthermore, it is used as an initiator in anionic polymerization of carbazolyl-substituted oxiranes. It catalyzes the reaction of hydrosilanes and heterocyclic compounds to give the silyl derivatives with evolution of hydrogen.
| PowerPoint PPT presentation | free to download
... a base removes the elements of an acid, HX, from the organic starting material. ... Removal of the elements HX is called dehydrohalogenation. ...
| PowerPoint PPT presentation | free to view
1] Dehydrohalogenation of alkenyl halides -C=C- -C C- 2HX. H X. i) CH3CH2CH ... 4] Addition of hydrogen halide. HC CR HX HHC=CX R HX HHHC CXXR. Br H ...
| PowerPoint PPT presentation | free to view
Vicinal dihalide dehalogenation. Alkenes via Alkyne Reduction ... E-2 Dehalogenation of Vicinal Dihalides. Periplanar anti-elimination ...
| PowerPoint PPT presentation | free to view
Markovnikov addition H ???????? electrophile ??????????? C ????? H ???????. Electrophile (E ) reagent ???????????????? ????????????????? ?????????????????? ...
| PowerPoint PPT presentation | free to download
3-Bromo-1-butene is formed faster than. 1-bromo-2-butene because allylic carbocations ... 1-Bromo-2-butene is more stable than. 3-bromo-1-butene because it has a ...
| PowerPoint PPT presentation | free to download
Title: Enthalpy Author: salim Last modified by: JB Created Date: 8/21/2003 6:50:14 AM Document presentation format: On-screen Show Company: Career Launcher
| PowerPoint PPT presentation | free to view
Substitution and Elimination Reaction of Alkyl Halides By: Ismiyarto, MSi * 4 18 18 9 9 21 21 2 4 6 6 8 11 6 2 6 26 29 32 H C C A B X Y H H H syn-Addition; Metal ...
| PowerPoint PPT presentation | free to download
Alkynes * * * * * * Structure sp hybridization Acidity of Terminal Alkynes Other strong bases that will ionize the terminal alkyne: Not KOH Stronger base Weaker base ...
| PowerPoint PPT presentation | free to download
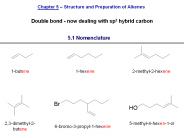
Chapter 5: Structure and Preparation of Alkenes: Elimination Reactions Alkenes (olefins) are hydrocarbons that contain a carbon-carbon double bond and are said to be ...
| PowerPoint PPT presentation | free to download
Alkynes. ???????????? ??????? ?????? IUPAC ????????????????? ??????????????? ... Addition reaction of 2,5-dimethyl-3-hexyne with two mole of hydrogen bromide ...
| PowerPoint PPT presentation | free to view
This group of compounds is a homologous series with the general molecular formula of CnH2n 2 The alkyne triple bond is ... of alkynes Slide 10 Reactions of ...
| PowerPoint PPT presentation | free to view
Steric. trans alkenes are more stable than cis alkenes ... Steric effect causes a large difference in stability. between cis and trans-(CH3)3CCH=CHC(CH3)3 ...
| PowerPoint PPT presentation | free to view
Substitution and Elimination Reaction of Alkyl Halides By: Ismiyarto, MSi ALKIL HALIDA Manfaat (Pestisida, Bahan Dasar Sintesis Alkohol, Alkena) Struktur (Metil ...
| PowerPoint PPT presentation | free to download
Title: No Slide Title Author: Youngstown State University Last modified by: Peter Norris Created Date: 8/30/2000 5:06:32 PM Document presentation format
| PowerPoint PPT presentation | free to download
exceedingly weak acids. Compound pKa. HF 3.2. H2O 16. NH3 36. 45. CH4 60. H2C. CH2 ... Prepare a solution containing sodium acetylide ...
| PowerPoint PPT presentation | free to download
(OR steric crowding at T.S. would destabilise a bimolecular transition state, ... (Why not consider the steric crowding at carbonium ion? ...
| PowerPoint PPT presentation | free to view
... 5.4 5.7 Cycloalkenes - trans not necessarily more stable than cis C-12 cis and trans ~ equal in energy Sterculic acid (natural product) ... Chemistry Other titles:
| PowerPoint PPT presentation | free to download
Arenes: compounds containing both aliphatic and aromatic parts. Alkylbenzenes Alkenylbenzenes Alkynylbenzenes Etc. Emphasis on the effect that one part has on the ...
| PowerPoint PPT presentation | free to download
If the group of highest priority on one carbon is on the same side as the group ... catalysis the hydrogen and alkene adsorb to the catalyst surface and then ...
| PowerPoint PPT presentation | free to download
The substitution reaction proceeds with fluorine instead of chlorine. ... octet is not possible for fluorine, an expanded octet intermediate is not likely. ...
| PowerPoint PPT presentation | free to view
Title: OC 2/e Ch 10 Alkynes Subject: Alkynes Author: Bill Brown Last modified by: Bill Brown Created Date: 7/18/1997 11:31:07 AM Document presentation format
| PowerPoint PPT presentation | free to download
Anti Addition of Hydrogen: Synthesis of trans-Alkenes Alkynes can be converted to trans-alkenes by dissolving metal ... to obtain TAXOL Anti-tumor, anti ...
| PowerPoint PPT presentation | free to view
Reactions of alkyne that are common to alkene are additions ... Tautomerism enol keto. Follow non-Markovnikov rule. Base catalyzed. 5/16/09. Chapter 8 - Alkyne ...
| PowerPoint PPT presentation | free to view
Reactions of alkyne that are common to alkene are additions ... Tautomerism enol keto. Follow non-Markovnikov rule. Base catalyzed. 8/11/09. Chapter 8 - Alkyne ...
| PowerPoint PPT presentation | free to view
To synthesize an isomeric mixture of alkenes from 'E2' base ... Cautiously add about 25 mL of water to the reaction flask and swirl to dissolve the solid salts. ...
| PowerPoint PPT presentation | free to view
1. reduction of alkene (addition of hydrogen) 2. reduction of an alkyl halide ... 3. dehalogenation of a vicinal dihalide. 4. reduction of an alkyne. reactions ...
| PowerPoint PPT presentation | free to download
Step 2: the alkenyl radical anion (a very strong base) abstracts a proton from ... a trans alkenyl anion is more stable than its cis isomer ...
| PowerPoint PPT presentation | free to view
* * Mercury acetate in the presence of water to give hydroxy-mercurial compounds which on reduction yields alcohol. Oxymercuration involves addition to C=C of OH and ...
| PowerPoint PPT presentation | free to download
SN1 and SN2 Reactions SN1 SN2 Rate =k[RX] =k[RX][Nuc:-] Carbocation intermediate? Y N Stereochemistry mix Inversion of configuration Rearrangement ~H, ~ CH3 possible
| PowerPoint PPT presentation | free to view
Chapter 32 Halogeno-compounds 32.1 Introduction 32.2 Nomenclature of Halogeno-compounds 32.3 Physical Properties of Halogeno-compounds 32.4 Preparation of Halogeno ...
| PowerPoint PPT presentation | free to view
Conjugation in Alkadienes and Allylic Systems conjugare is a Latin verb meaning
| PowerPoint PPT presentation | free to view
Synthesis of trans-Alkenes by Anti Addition Alkynes can be converted to trans-alkenes by dissolving ... * TAXOL the from Pacific Yew tree a 100-year old yew ...
| PowerPoint PPT presentation | free to download
... methyl bromide methanol ethyl acetate acetate and ethanol at ambient pH, reactant and product concs, ...
| PowerPoint PPT presentation | free to view
Prepare a solution containing sodium acetylide ... Sodium amide. is a stronger base than sodium hydroxide. NH3. NaNH2. HC. CH. NaC. CH. H2N ...
| PowerPoint PPT presentation | free to view
Chapter 6 Ionic Reactions---Nucleophilic substitution and elimination reactions of alkylhalides (
| PowerPoint PPT presentation | free to view
7.11 Chiral Molecules with Two Chirality Centers ... Alkynes
| PowerPoint PPT presentation | free to download
Nucleophile weak lewis base (neutral) Strong lewis base, high conc. ... Substitution: preferred with primary halides and polarizable, small. nucleophiles ...
| PowerPoint PPT presentation | free to view
... for cyclic alkenes Structure Of Alkenes * Trigonal planar 2s2 2p3 3 x sp2 2p sp2 Hybridization Of ... * Preparation Of Alkenes 1- Dehydration of ...
| PowerPoint PPT presentation | free to view
Aldehydes are easily oxidized, ketones are not. ... CH3CH2CH2CH=O KMnO4, etc. ... 3. Dehalogenation of a vicinal dihalide. 4. Reduction of an alkyne ...
| PowerPoint PPT presentation | free to view
With diastereomerically pure vicinal dihaloalkones, a single haloalkene product ... through an isolatable intermediate vicinal dihaloalkene, to the tetrahaloalkane. ...
| PowerPoint PPT presentation | free to view
... Lone Pair on Bromine Stabalizes Carbocation and Forms Cyclic Bromonium Ion Step 2; Bromide Ion Must Attack from Oppositte Side of Cyclic Bromonium Ion ...
| PowerPoint PPT presentation | free to view
... (dehydration) require strong acids (sulfuric acid, 50 C) * Part 2 - Reaction of Alkenes These reactions react alkenes to form a series of alkane products. * ...
| PowerPoint PPT presentation | free to view
Lecture 10 Alkene Reactions
| PowerPoint PPT presentation | free to view
Species with higher electronegativity have tightly held ... Bimolecular or Unimolecular refers to the number of specie(s) involved at the transition state. ...
| PowerPoint PPT presentation | free to view
... in synthesis owing to their greater selectivity (see J. Vyvyan) ... We choose other metals for different degrees of reactivity and for greater selectivity. ...
| PowerPoint PPT presentation | free to view
Step 4: Tautomerism gives the keto form. Addition of H2O: hydration ... Steps 6 and 7: Loss of Hg2 gives an enol; tautomerism of the enol gives the ketone. ...
| PowerPoint PPT presentation | free to view
Title: OC 2/e Ch 8 S & E Subject: Alkyl halides, etc. Author: Bill Brown Last modified by: Bill Brown Created Date: 7/15/1997 1:28:14 PM Document presentation format
| PowerPoint PPT presentation | free to download