C6h6 PowerPoint PPT Presentations
All Time
Recommended
Title: 1 Author: zxm Last modified by: zxm Created Date: 2/16/2005 2:41:43 PM Document presentation format: Other titles
| PowerPoint PPT presentation | free to view
Arenes and Aromaticity Aromatic Compounds Contain Benzene; C6H6 22 26 29 31 31 31 31 16 14 18 25 26 37 30 30 18 Polycyclic Aromatic Hydrocarbons (PAH) n =2 ...
| PowerPoint PPT presentation | free to view
... (C6H5)2 hn C6H6 phenone Isomeric : N = N hn C6H5 C6H5 (cis - azobenzene) (tran - azobenzene) N = N C6H5 C6H5 Decomposition: CH3-C-CH3 ...
| PowerPoint PPT presentation | free to view
Benzene and Aromaticity Bonding in Benzene Proposed Benzene Structures C6H6 reacts with HBr to form one isomer of C6H5Br Other Conjugated Hydrocarbon Rings Annulenes ...
| PowerPoint PPT presentation | free to download
Catalytic Reforming of Petroleum Converts alkanes and cycloalkanes into aromatic hydrocarbons C6H14 C6H6 + 4H2 500 oC, 10 ...
| PowerPoint PPT presentation | free to view
How do properties of solutions differ from properties of the solute and solvent? ... torr. Ptoluene = 22 torr. Answer: 1 mol C6H6 , 1 mol C7H8, 37.5 torr C6H6, ...
| PowerPoint PPT presentation | free to view
Chapter 12.5 Aromatic Hydrocarbons * * Aromatic Compounds and Benzene Aromatic compounds contain benzene. Benzene, C6H6 , is represented as a six carbon ring with 3 ...
| PowerPoint PPT presentation | free to view
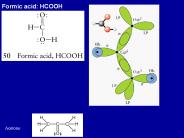
Formic acid: HCOOH. Acetone. Benzene C6H6. Resonance structures; each point corresponds to a CH ... Each C is sp2 hybridized, one of the sp2 forming a s-bond ...
| PowerPoint PPT presentation | free to download
Chapter 11: Arenes and Aromaticity 11.1: Benzene - C6H6 11.2: Kekul and the Structure of Benzene Kekule benzene: two forms are in rapid equilibrium
| PowerPoint PPT presentation | free to download
A mass spectrometer bombards a substance under investigation with an electron ... Fragmentation of Benzene. Benzene (C6H6) has substantial m/z 79 and m/z 80 peaks. ...
| PowerPoint PPT presentation | free to view
identify the number of carbon atoms in the longest continuous chain and use the ... Benzene C6H6. 11/10/09. 17. Ethane, C2H6. Molecular Formula #1 ...
| PowerPoint PPT presentation | free to download
Benzene and Aromatic Compounds Benzene (C6H6) is the simplest aromatic hydrocarbon (or arene). Benzene has four degrees of unsaturation, making it a highly ...
| PowerPoint PPT presentation | free to view
... larger the lattice E Ex: Bond length (determined by ionic radii) - Smaller the radii, ... (C6H6) 9.1-2 Molecular Shapes and VSEPR Theory Draw LDD.
| PowerPoint PPT presentation | free to download
Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6. ... Pyridine. Heterocyclic aromatic compound. ...
| PowerPoint PPT presentation | free to view
Benzene and Its Derivatives Chapter 4 Kekule s Structure of Benzene (A)The bonds in C6H6 are something between single and double, which gives it different chemical ...
| PowerPoint PPT presentation | free to view
Mechanisms of conversion of heavy hydrocarbons in biogas ... Pyrene. 0.12. C6H6O. Penol. 17.86. C10H8. Naphthalene. 1.28. C7H8. Toluene. 67.56. C6H6. Benzene ...
| PowerPoint PPT presentation | free to download
1,2,4-trifluorobenzene(TFB) : C6F3H3. hexafluorobenzene(HFB) : C6F6. Condition ... Time-of-Flight SIMS. DSC. Results and Discussion. C6H6. Aromatic ring mode ...
| PowerPoint PPT presentation | free to view
Stoichiometry Test Review #1. Hexane (C6H14) burns in oxygen to give carbon ... If 215 g of hexane is mixed with 215 g of oxygen, what mass of carbon dioxide is ...
| PowerPoint PPT presentation | free to view
R3P, (RO) 3P, R3As, C2H4, C6H6. Hard & Soft Bases. Hard ligands ... R3P, RNC, CN-, CO, R-, H-, I-, S2O32-, (RS)2PO2-, (RO)2P(O)S ...
| PowerPoint PPT presentation | free to view
Click a box below to go to the mechanism Click here for advice Homolytic Free Radical Substitution Free Radical Addition Heterolytic Electrophilic Addition
| PowerPoint PPT presentation | free to download
Probing very long-lived excited electronic states of molecular ... The inset shows the details of the electrode assembly. J. Chem. Phys. 113, 9532 (2000) ...
| PowerPoint PPT presentation | free to view
Benzene Part 1 ELECTROPHILIC SUBSTITUTION Theory The high electron density of the ring makes it open to attack by electrophiles Addition to the ring would upset ...
| PowerPoint PPT presentation | free to view
ARENES CONTENTS Prior knowledge Structure of benzene Thermodynamic stability Delocalisation Electrophilic substitution Nitration Chlorination Friedel-Crafts reactions
| PowerPoint PPT presentation | free to download
Title: Slide 1 Author: James A. Sy Last modified by: James A. Sy Created Date: 12/10/2006 6:16:34 PM Document presentation format: On-screen Show Company
| PowerPoint PPT presentation | free to download
Preview Lesson Starter Objective Stoichiometry Definition Reaction Stoichiometry Problems Mole Ratio Stoichiometry Calculations
| PowerPoint PPT presentation | free to view
Preview Lesson Starter Objective Stoichiometry Definition Reaction Stoichiometry Problems Mole Ratio Stoichiometry Calculations
| PowerPoint PPT presentation | free to view
Title: PowerPoint Presentation Author: Troy Wood Last modified by: Emilcomp Created Date: 10/12/2004 3:22:25 PM Document presentation format: On-screen Show
| PowerPoint PPT presentation | free to download
The Molecular Formula The empirical formula CH has a molar mass of 13 g/mol. Acetylene, ... Determining a Molecular Formula The empirical formula for ribose is CH2O.
| PowerPoint PPT presentation | free to view
The merging of several atomic orbitals to form the same total number of hybrid orbitals. ... http://andromeda.rutgers.edu/~huskey/images/ethylene_bonding.jpg ...
| PowerPoint PPT presentation | free to view
Title: Slide 1 Author: Hanan E. Abdou Last modified by: Wendy Created Date: 8/5/2005 3:44:21 AM Document presentation format: On-screen Show Company
| PowerPoint PPT presentation | free to download
PowerPoint Presentation ... Air
| PowerPoint PPT presentation | free to download
List the elements in the molecule Carbon Chlorine Lewis ... take 3 Of course, real molecules are 3-dimensional, not flat as shown in a structural diagram. For ...
| PowerPoint PPT presentation | free to download
Electrophilic Substitution. Nucleophilic Addition. Nucleophilic ... 1. Formation of the electrophile (an acylium ion) AlCl3. Cl AlCl3. With ethanoyl chloride ...
| PowerPoint PPT presentation | free to view
Internal Combustion Engine Chapter 4 Thermochemistry and Fuels Thermochemistry ...
| PowerPoint PPT presentation | free to view
Title: Slide 1 Author: OPO Lab Last modified by: Geoffrey A. Blake Created Date: 5/3/2004 1:24:55 AM Document presentation format: Custom Company
| PowerPoint PPT presentation | free to download
benzene. Example. How is the Degree of Unsaturation of a. hydrocarbon containing halogen, ... benzene ring - 4 DU. carbonyl - 1 DU. C8H6Cl2O3. 2,4 ...
| PowerPoint PPT presentation | free to view
Industrial Chemistry
| PowerPoint PPT presentation | free to view
Aliphatic (fatty) Aromatic (fragrant) Open-chain cyclic compounds ... cyclic clouds of delocalized p electrons above and below the ...
| PowerPoint PPT presentation | free to view
Thermochemistry Unit Section 16.2
| PowerPoint PPT presentation | free to view
The structure of benzene L.O.: Explain the structure of benzene. Use evidence from enthalpies of hydrogenation to illustrate aromatic stability. * Work in groups of 4.
| PowerPoint PPT presentation | free to view
Classes of Hydrocarbons
| PowerPoint PPT presentation | free to download
Standardized Test Preparation Preview Multiple Choice Short Answer Extended Response
| PowerPoint PPT presentation | free to view
Polycyclic aromatic hydrocarbons (PAHs) are the most abundant free organic ... Discharge plasma is guided to skimmer. Ions are extracted into TOF MS by pulser. ...
| PowerPoint PPT presentation | free to download
Click a box below to go to the mechanism Click here for advice AS Free Radical Substitution Electrophilic Addition Nucleophilic Substitution (nitrile hydrolysis reaction)
| PowerPoint PPT presentation | free to view
... q0 rod set _____ A novel chemical reactor suited for studies of biophysical chemistry: construction and evaluation of a ... Catalysis N2O N2 CO M+ MO+ ... Design ...
| PowerPoint PPT presentation | free to view
Standardized Test Preparation Preview Multiple Choice Short Answer Extended Response Multiple Choice 1. In stoichiometry, chemists are mainly concerned with A. the ...
| PowerPoint PPT presentation | free to download
* * * * Figure 15.8: Pyridine and pyrimidine are nitrogen-containing aromatic heterocycles with electron arrangements much like that of benzene.
| PowerPoint PPT presentation | free to download
Enthalpy Specific Heat Calorimetry Hess Law * Additional energy must be put in to vaporize H2O * Calorimeters used to measure heat transfer Can be either constant ...
| PowerPoint PPT presentation | free to view
Stoichiometry Review Guide What is stoichiometry? Multiple Choice In stoichiometry, chemists are mainly concerned with A. the types of bonds found in compounds.
| PowerPoint PPT presentation | free to view
Title: PowerPoint Presentation Author: ionchem Created Date: 7/17/2006 1:57:27 PM Document presentation format: On-screen Show Other titles: Times New Roman Arial WP ...
| PowerPoint PPT presentation | free to view
The Morphology of Laser-Synthesized Carbon Nano-Fillers: the Influence on Polymer-Based Composites Lavinia Gavrila-Florescu1*, Ion Sandu1 1National Institute for ...
| PowerPoint PPT presentation | free to download
Bomb Calorimetry constant volume often used for combustion reactions heat released by reaction is absorbed by calorimeter contents need heat capacity of calorimeter
| PowerPoint PPT presentation | free to download
Combustion of propane (C3H8) gas to form CO2(g) ... of 1 mole of propane DHorxn = -2220 kJ ... Liquid petroleum gas (LPG) - propane - also used as a fuel ...
| PowerPoint PPT presentation | free to download
... Calculations Stoichiometric Calculations Stoichiometric calculations Limiting Reactants How Many Cookies Can I Make? How Many Cookies Can I Make?
| PowerPoint PPT presentation | free to view
Application of COLTRIMS to Study Collision Induced Dissociation of ... a) P.J. Richardson, J.H.D. Eland and P. Lablanquie, Organic Mass Spectrometry 21 ...
| PowerPoint PPT presentation | free to download