R, R - PowerPoint PPT Presentation
1 / 145
Title: R, R
1
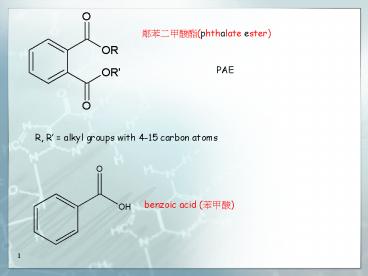
PAE
R, R alkyl groups with 4-15 carbon atoms
2
Used as plasticiser Potential carcinogen New car
smell
R, R alkyl groups with 4-15 carbon atoms
2
3
Used as plasticiser Potential carcinogen Endocrine
disruption New car smell
3
4
dibutyl benzene-1,2-dicarboxylate
butyl pentyl benzene-1,2-dicarboxylate
5
isophthalic acid meta-phthalic acid ?????
terephthalic acid para-phthalic acid ?????
benzene-1,3-dicarboxylic acid
benzene-1,4-dicarboxylic acid
6
polyester
(terylene)
7
Polyethylene terephthalate
PET or PETE
8
Isomerism
9
Isomerism
Occurs when certain compounds, having the same
molecular formula, exist in different forms.
Isomers
Compounds having the same molecular formula but
different linkages or spatial arrangements of
atoms
10
Isomerism
- Two main types of isomerism
- Structural isomerism
- different linkages of atoms.
- Stereoisomerism
- different spatial arrangements of atoms
11
Isomerism
- Two main types of isomerism
- Structural isomerism
- different linkages of atoms.
Same molecular formula Different structural
formulae
12
Isomerism
- Stereoisomerism
- different spatial arrangements of atoms
Same molecular formula structural formulae
13
Categories of Isomerism
14
Categories of Isomerism
Stereoisomerism
Optical isomerism
Geometrical isomerism
Diastereoisomerism
Enantiomerism
Optical isomers that are NOT mirror images of
each other
Optical isomers that are mirror images of each
other
15
Structural Isomerism
16
1. Chain isomerism
due to the presence of different carbon
skeletons.
17
C6H14
p.2, Q.2
1. Chain isomerism
18
1. Chain isomerism
- Different physical properties
- b.p. Straight-chain gt branched-chain
- Reason - larger surface area and thus
stronger v.d.w. forces
19
1. Chain isomerism
- Different physical properties
m.p. Symmetry of molecule ? ? Packing
efficiency ? ? m.p. ?
20
1. Chain isomerism
- Different physical properties
Notes on Bonding and Structure p.81, Q.61
21
Chain isomers have different physical properties.
What about their chemical properties?
Would they be different as well?
Chain isomers have similar chemical properties
because they have the same functional groups.
22
2. Position isomerism
Same carbon skeleton Different positions of
functional groups
23
2. Position isomerism
e.g. Butan-1-ol and butan-2-ol (molecular
formula C4H10O)
24
Q.15
Are they position isomers ?
They are chain isomers
25
3. Functional Group Isomerism
Due to the presence of different functional groups
26
Functional Group Isomerism
C2H6O
27
Functional Group Isomerism
C3H6O
28
Functional Group Isomerism
C3H6O2
29
Chain isomers
C5H10
30
(No Transcript)
31
4. Metamerism
Occurs when the functional group (-oxy or
carbonyl) interrupts the main carbon skeleton at
different positions
32
4. Metamerism
e.g. Methoxypropane and ethoxyethane (molecular
formula C4H10O)
33
4. Metamerism
e.g. Pentan-2-one and pentan-3-one (molecular
formula C5H10O)
can also be considered as position isomers
34
Position isomers
C4H10O
Position isomers
35
Position isomers
C4H10O
Metamers
36
5. Tautomerism
Occurs when functional group isomers are in
dynamic equilibrium with each other.
37
5. Tautomerism
ethenol
ethanal
enol keto tautomerism
38
5. Tautomerism
ethenol
ethanal
Enol is a structure with OH attached directly to
doubly-bonded C
39
5. Tautomerism
But-2-en-1-ol
Not an enol
Does not exhibit tautomerism
40
Q.16
Which one, the enol form or the keto form, is the
stronger acid ?
The enol form is the stronger acid because the H
attached to the more electronegative O can be
released as H more easily.
41
Q.16
More stable
Less stable
stronger acid weaker acid
The equilibrium position lies to the right
42
Stereoisomerism
43
Stereoisomerism
occurs when compounds having the same structural
formula show different spatial arrangements of
atoms.
44
Stereoisomerism
- Two categories of stereoisomerism
- Geometrical isomerism
- Optical isomerism
45
Stereoisomerism
Geometrical Isomerism
arises from restricted rotation about a CC
double bond.
46
cannot be inter-converted at lower temperatures
47
maximum overlap of pz orbitals
minimum overlap of pz orbitals
Rotation about the axis of a double bond through
an angle of 90o results in the breaking of the p
bond
48
Geometrical Isomerism - Criteria
where a ? b and c ? d
49
E/Z notation
If there are three or four different groups
attached to the Cs of CC double bond E/Z
notation rather than the cis/trans notation is
used to name the stereoisomers of a molecule. E
in opposition to ? trans Z together ? cis
http//en.wikipedia.org/wiki/Cahn-Ingold-Prelog_pr
iority_rule
50
Q.17
Rotate the molecular plane w.r.t. the axis by 180?
The same a b
51
Q.17
The same a b and c d
52
Properties of Geometrical Isomers
They have significantly different physical
properties
53
Zero net dipole moment
van der Waals forces cis gt trans b.p. depends
on v.d.w. forces ONLY ? b.p. cis gt trans
54
more symmetrical less symmetrical
Two planes of symmetry
Three planes of symmetry
55
more symmetrical less symmetrical
higher packing efficiency
lower packing efficiency
m.p. is more affected by symmetry of molecules. ?
m.p. trans gt cis
55
56
2.
m.p. 130?C ltlt 290?C
57
(No Transcript)
58
weaker v.d.w. forces lower packing
efficiency ? much lower m.p.
59
Some geometrical isomers exhibit significantly
different chemical properties
1.
K1 1.4?10?2 mol dm?3 gtgt 9.3?10?5 mol dm?3
K2 8.0?10?7 mol dm?3 lt 3.6?10?5 mol dm?3
60
Formation of intra-molecular hydrogen bond helps
to draw electrons from the OH
- Weakening O H
- Ease of release of H ?
? K1 cis gt trans
61
Furthermore, the -ve charge produced is
stabilized by forming intra-molecular hydrogen
bond.
In other words, the conjugate base is stabilized
62
significant repulsion
repulsion is minimized
63
Q.18
Solubility in water (gram of solute per 100 g of
water at 25C)
78.8 0.7
Intra-molecular hydrogen bonds in cis-isomer
reduce the extent of formation of intermolecular
hydrogen bonds. Thus, water molecules can
separate the acid molecules of the cis-isomer
more easily ? solubility of the cis-isomer in
water is higher
64
2.Thermal dehydration of butenedioic acid
150C
250C
-H2O
-H2O
breaking and forming of ? bond by rotating by
180 about the axis of the CC double bond
65
plane-polarized light
66
Optically active substance can rotate the plane
of polarization of plane-polarized light Measured
by a polarimeter.
67
Dextrorotatory() clockwise (to the
right) Laevorotatory(-) anti-clockwise (to the
left)
Is the above sample dextrorotatory ? No. It is
laevorotatory
68
Optical activity arises from lack of
symmetry asymmetry (???) chirality (??)
E.g. A molecule with an sp3 carbon atom bonded
to FOUR different groups
69
The molecule has no plane or axis or center of
symmetry. ? It is asymmetric
70
mirror
It is not superimposable with its mirror image ?
It exhibits chirality
71
(No Transcript)
72
Enantiomers (?????)
Mirror images of each other Non-superimposable
with each other Rotate plane-polarized light to
the same extent but in opposite directions
73
Enantiomers (?????)
The direction of optical rotation cannot be
predicted from the structural formulae. It can
only be determined experimentally.
74
Enantiomers (?????)
() or (-)butan-2-ol
75
C is known as the chiral center or asymmetric
centre
76
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp3 hybridized C atoms each bonded to THREE
identical H atoms ? Not asymmetric
77
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp2 hybridized C atoms are NOT chiral centers
78
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
The molecule can be cut into two identical halves
by the molecular plane It has a plane of
symmetry It is NOT asymmetric
79
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp3 hybridized C atom bonded to three identical H
atoms ? Not asymmetric
80
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp3 hybridized C atom bonded to two identical Br
atoms ? Not chiral
81
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp3 hybridized C atom bonded to two identical H
atoms ? Not chiral
82
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp3 hybridized C atom bonded to two identical H
atoms ? Not chiral
83
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp3 hybridized C atoms bonded to three identical
H atoms ? Not chiral
84
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp3 hybridized C atom bonded to two identical CH3
groups ? Not chiral
85
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
86
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
Asymmetric Optically active
() or (-)
sp3 hybridized C atom bonded to four different
groups
87
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp3 hybridized C atoms bonded to three identical
H atoms ? Not chiral
88
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
sp3 hybridized C atoms bonded to four different
groups
89
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
90
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
91
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
Two chiral centers but optically inactive
92
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
The molecule has a plane of symmetry ? Not
asymmetric
93
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
Meso compound
The optical rotation caused by the left chiral
center is cancelled by the optical rotation
caused by the right chiral center.
internal cancellation
94
A meso compound is a compound whose molecules
contain 2 or more asymmetric atoms but is
optically inactive.
95
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
The same
Two identical groups attached to the carbon ? Not
asymmetric
96
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
The molecule has a plane of symmetry ? Not
asymmetric
97
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
Not the same
Four different groups attached to the carbon ?
asymmetric
98
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
Optically inactive A meso compound
99
Q.19 Indicate the asymmetric carbon atom(s) in
each of the following molecules.
Which of them is/are optically
active ?
Optically inactive A meso compound
100
Properties of enantiomers
Identical physical properties except their
optical activities.
101
Properties of enantiomers
Identical chemical properties except their
reactions with optically active substances.
102
Racemic mixture (racemate)
An equimolar mixture of a pair of
enantiomers Opically inactive The clockwise
rotation caused by the ()isomer is cancelled by
the anti-clockwise rotation caused by the
(-)isomer
external cancellation
103
Racemic mixture (racemate)
- The ()isomer and (-)isomer are different
structures - One serves as the impurity of the other in a
racemic mixture. - A racemic mixture has a lower m.p. than its
components.
104
Identification of solid unknowns
- By melting point determination
- Determine the m.p. of the unknown and compare
the result with the m.p. of known compounds
105
Identification of solid unknowns
2. By mixed melting point determination
(more reliable) Mix the unknown solid with a
pure solid with known m.p. Determine the m.p. of
the mixture and compare the result with the m.p.
of the known solid.
106
Q.20
Different structures may have the same melting
point. In mixed melting point determination,
different structures with the same melting point
behave as impurities to each other.
107
Structure determination of organic compounds
from (i) molecular formula (ii) reactions of
functional groups (chemical properties) (iii)
physical properties (iv) Infra-red / Mass spectra
?
108
CxHyOwNz X(halo) ? H
- Acyclic with single bonds ONLY
- IOU 0
109
CxHyOwNz
- Acyclic with single bonds ONLY
- IOU 0
110
CxHyOwNz
- Acyclic with single bonds ONLY
- IOU 0
111
C2H6ClN
112
2. Acyclic with ONE double bond IOU 1
113
2. Acyclic with ONE double bond IOU 1
114
2. Acyclic with ONE double bond IOU 1
115
E/Z
CH3O2N
()/(-)
116
3. With ONE triple bond IOU 2
117
4. One cyclic IOU 1
118
THREE CC bonds ONE ring IOU 4
119
Q.21
sp hybridized carbon atoms in triple bond should
take a linear shape.
120
Q.21
sp hybridized carbon atoms in triple bond should
take a linear shape.
121
Q.21
The carbon atom at the 2nd position, C2, is sp
hybridized ? it takes a linear
shape. Side-way overlap between py orbitals of C1
and C2 gives a ? bond between C1 and C2. Side-way
overlap between pz orbitals of C2 and C3 gives
another ? bond between C2 and C3. Systems with
adjacent CC bonds are less stable than
conjugated systems due to a lack of
delocalization of ? electrons.
122
7 isomers !
Q.22(a) C3H6O
? One CO bond or one CC bond
propanone
propanal
123
Q.22(a)
(1E)-prop-1-en-1-ol (1Z)-prop-1-en-1-ol Prop-1-e
n-2-ol
prop-2-en-1-ol
methoxyethene
CC OH
124
24 isomers !
Q.22(b) C3H6O2
? One CO bond or one CC bond
125
Q.22(b)
Carboxylic acid or ester
propanoic acid
methylethanoate ethylmethanoate
126
CC two -OH
Prop-1-ene-1,1-diol
Prop-2-ene-1,1-diol
Q.22(b)
126
127
CC two -OH
(1Z)-prop-1-ene-1,2-diol
(1E)-prop-1-ene-1,2-diol
Q.22(b)
128
Q.22(b)
Prop-2-ene-1,2-diol
129
Q.22(b)
(1Z)-prop-1-ene-1,3-diol
(1E)-prop-1-ene-1,3-diol
130
Q.22(b)
CC OH -O-
1-methoxyethenol
(Z)-2-methoxyethenol
(E)-2-methoxyethenol
131
Q.22(b)
CC oxy -OH
(ethenyloxy)methanol
132
Q.22(b)
CO OH
1-hydroxypropanone
Ketone alcohol ? ketol
133
Q.22(b)
Aldehyde alcohol ? aldol
3-hydroxypropanal
() or (-)2-hydroxypropanal
134
Q.22(b)
Aldehyde oxy
2
2-methoxyethanal
135
Q.22(b)
ene peroxy
(methylperoxy)ethene
Ethenyl methyl peroxide
136
Q.22(b)
ene peroxy
(1E)-prop-1-en-1-yl hydroperoxide
(1Z)-prop-1-en-1-yl hydroperoxide
137
Q.22(b)
prop-1-en-2-yl hydroperoxide
138
Q.22(c)
C5H8
Alkyne 3 Diene 8
139
(No Transcript)
140
pentadienes
141
2-methylbuta-1,3-diene
3-methylbuta-1,2-diene
142
C5H8
Cycloalkene 10 Bicyclic 2 Spiro 1
143
(No Transcript)
144
(No Transcript)
145
bicyclo2.1.0pentane
bicyclo1.1.1pentane
spiro2.2pentane