Thermodynamics of Ionic Crystal Formation - PowerPoint PPT Presentation
1 / 15
Title:
Thermodynamics of Ionic Crystal Formation
Description:
Used to determine electron affinity when all other reactions experimentally known ... Mg and Silicate layers differ in size leading to curling fibrous structure ... – PowerPoint PPT presentation
Number of Views:218
Avg rating:3.0/5.0
Title: Thermodynamics of Ionic Crystal Formation
1
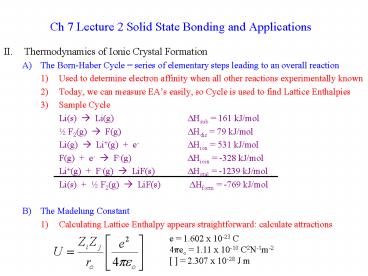
Ch 7 Lecture 2 Solid State Bonding and
Applications
- Thermodynamics of Ionic Crystal Formation
- The Born-Haber Cycle series of elementary steps
leading to an overall reaction - Used to determine electron affinity when all
other reactions experimentally known - Today, we can measure EAs easily, so Cycle is
used to find Lattice Enthalpies - Sample Cycle
- Li(s) ? Li(g) DHsub 161 kJ/mol
- ½ F2(g) ? F(g) DHdis 79 kJ/mol
- Li(g) ? Li(g) e- DHion 531 kJ/mol
- F(g) e- ? F-(g) DHioin -328 kJ/mol
- Li(g) F-(g) ? LiF(s) DHxtal -1239 kJ/mol
- Li(s) ½ F2(g) ? LiF(s) DHform -769
kJ/mol - The Madelung Constant
- Calculating Lattice Enthalpy appears
straightforward calculate attractions
e 1.602 x 10-23 C 4peo 1.11 x 10-10 C2N-1m-2
2.307 x 10-28 J m
2
- Problem Long-range interactions change the
Lattice Enthalpy - NaCl Na has 6 Cl- nearest neighbors at ro ½ a
(accounted for in equation) - Na also has 12 Na neighbors at r 0.707 a (not
accounted for) - Na also has many other Na and Cl- neighbors at
longer distances - M Madelung Constant takes into account all
attractions - Born-Mayer Equation incorporates Madelung
Constant as well as accounting for repulsions
(much more complicated than attractions) - r constant 30 pm works well
- Increase in charge causes corresponding increase
in Lattice Enthalpy - 2/2 charges would give 4 x Lattice Enthalpy
3
- Solubility, Size, and HSAB
- We can use a Born-Haber Cycle to calculate
dissolution energies - AgCl(s) ? Ag(g) Cl-(g) DHLattEnth 917
kJ/mol - Ag(g) H2O ? Ag(aq) DHsolvation -475
kJ/mol - Cl-(g) H2O ? Cl-(aq) DHsolvation -369
kJ/mol - AgCl(s) H2O ? Ag(aq)
Cl-(aq) DHdissolution 73 kJ/mol - Factors effecting solubility
- Size
- Small ions have strong attractions larger ions
have small attractions - Large ions may have more water molecules
surrounding them - Large/Large and Small/Small salts are less
soluble than Large/Small - LiF CsI lt LiI CsF 1. Lattice Energy 2.
HSAB
4
- Molecular Obitals and Band Structure
- Band Formation
- Overlap of 2 AOs gives 2 MOs
- Overlap of n AOs gives n MOs
- Solids have very large values of n, sometimes
multiples of N - Band many closely spaced MOs of nearly
continuous energy - Valence Band highest occupied band (HOMO)
- Conduction Band next highest empty band (LUMO)
- Insulator large energy gap between Valence and
Conduction bands - Electrons cant move through material
- Electron motion is what allows conduction of
electricity and heat - Conductor partly filled Valence and Conduction
bands (Most Metals) - Little energy required for electron movement
holes
Insulator Conductor w/ no
Voltage Conductor with applied Voltage
5
- Density of State concentration of E levels in a
Band N(E) - Temperature Effect on Conductors (Metals)
- Vibrations increase as temperature increases
- Vibrations interfere with electron movement, slow
conductance (increase resistance) - Semiconductors full Valence Band, Empty
Conductance Band, close together - Energy gap lt 2 eV
- Si, Ge are common pure substances that are
semiconductors - At low temperature they are insulators (not as
good as true insulators) - At higher temperature they are conducturs (not as
good as true conductors) - Opposite temperature effects as metals (true
conductors)
6
- Doped Semiconductors
- We can closely control on/off properties of
semiconductors this has led to the entire field
of solid state electronics (computers) - Intrinsic Semiconductor pure form is
semiconductor (Si, Ge) - Doped Semiconductor small amount of impurity
effects semiconduction - n-type semiconductor dopant has more e- than
host (P in Si) - p-type semiconductor dopant has less e- that
host (Al in Si) - Careful doping results in tailored materials
- Fermi Level EF Energy at which e- equally
likely to be in either band - Intrinsic EF about in middle of gap
- n-type EF raised above new band
- p-type EF lowered below new band
n-type semiconductor p-type semiconductor
7
- Devices Using Semiconductors
- p-n Junction
- Diode device where current flows only in one
direction - At equilibrium a few e- have moved from n-type to
p-type (n is , p is -) - EF is at same level in n-type and p-type at
equilibrium - Apply negative pot. to n-type and positive pot.
to p-type - Forward Bias
- Extra electrons allow e- in n-type to flow to
p-type holes - Holes move to junction from p-type side
- Current flows readily
- Reverse Bias holes and electrons move away from
juction no current flows
????
????
8
- Photovoltaic Cells
- A p-n Junction with the energy gap hn of a
light source - Light causes e- to jump to p-type even under
reverse bias conditions - Light detector Calculator battery
- LED Light Emitting Diode
- A p-n Junction with forward bias
- Electrons move from n to p and release energy in
the form of light - Lower temp increases efficiency by decreasing
vibrations - 4) Laser Light Amplification by Stimulated
Emission of Radiation - LED with large band gap in p-type to prevent e-
from escaping middle band
9
- Superconductivity
- The Phenomenon
- The conductivity of some metals changes abruptly
- around 10 K (Critical Temperature TC)
- 2) Superconductor no resistance to e- flow
- Kammerling and Onnes 1911 discover
- for Hg cooled by liquid He
- Major use today is superconducting magnets for
NMR - Low Temperature Alloys
- Type I Superconductors are often Nb-Ti alloys
- Abrupt change between superconducting and normal
conduction - Meissner Effect no magnetic flux can enter
superconductor below TC - Floating Magnets demonstration
- Strong magnetic fields destroy superconduction
above HC - Nb3Ge has highest TC 23.3 K for this type of
superconductor - Type II Superconductors work below TC1 and are
complex between TC1 and TC2 - Some magnetic flux can enter them in complex
region (Floating Magnets)
10
- Theory Cooper Pairs
- 1950s Bardeen, Cooper, and Schrieffer propose
BCS Theory - Electrons travel through superconductors in pairs
(Cooper Pairs) - Opposite spin electrons are slightly attracted at
low temperatures - As one e- moves past nucleus in metal, the next
nucleus attracts it - This increases the charge density, so the second
e- of the pair is attracted to - Cooper pairs move through metal like a wave
- Lattice helps push/pull e- through no resistance
because energetically favorable - When T gt TC the thermal motion of the nuclei
disrupt the wave - High Temperature Superconductors
- (La2-xSrX)CuO4 found to have TC 30 K in 1986
- YBa2Cu3O7 found to have TC 92 K in 1987
- Type II superconductors
- Cool with N2(l) cheap, bp 77K instead of
He(l) expensive, bp 3K - Ceramic brittle cant easily make into wire,
etc - Structure copper oxide planes and chains
- Theory BCS Theory applies, but not completely
understood
11
- Bonding in Ionic Crystals
- Simplest Idea hard spheres with only
electrostatic interactions - Deviations form Simple Idea
- Ionic size is difficult to measure
- Pauling Li 60 pm (from calculations)
- Shannon Li 90 pm (from crystal structures)
- Sharing of electrons back from anion effects the
size of the cation - Covalent Interactions very important as well ZnS
is strongly covalent - Complex theory involving MOs Crystal Field
Theory (Chapter 10) - Imperfections in Solids
- Crystal Growth
- Slowly grown crystals are more perfect
- Quickly grown crystals are usually made up of
many small crystals which have run into each
other grain boundaries - Even perfect crystals have impurities and
vacancies - Vacancies and Self-Interstitials
- Vacancy missing atom, ion, or molecule in the
crystal simplest imperfection - More formed at higher T, but only 1/10,000 even
near the melting point - Effect is small localized in one unit cell
and/or layer of the crystal
12
- Self-Interstitials atoms/ions/molecules in the
wrong place - Effect is felt for several layers of the crystal
- Usually much rarer than vacancies
- Substitutions one element/ion in place of the
expected element/ion - Common occurrence leading to a Solid Solution
- Ni/Cu similar in size and electronegativity both
have (fcc) structure - Mixtures stable in any proportion alloy
- Random arrangement of atoms since they are so
similar - Small atoms in holes between larger ones
- Occupying a hole usually has small effect on the
rest of the structure - May have large effect on properties of the
material (C in Fe makes steel) - If impurity is large than hole lattice strain or
new solid phase - Dislocations
- Atoms in one layer dont match up with the next
- Distances and angles effected for several layers
in each direction - Screw Dislocation one layer shifted a fraction
of unit cell - Rapid growth location (more attraction into
solution) helical result
13
- The Silicates
- Abundance
- O, Si 80 of Earths Crust
- Many compounds and minerals formed, some
industrially important - Silica SiO2
- Three crystalline forms Quartz (low T form),
Tidymite, and Cristobalite - Molten SiO2 often forms glasses instead of
crystalline forms - Glass disordered solid structure
- Actually a solution that continues to flow but
very slowly - SiO4 tetrahedra in all crystal forms with SiOSi
angle 143.6o - Quartz
- Most common form
- Helical chains of SiO4 tetrahedra make it chiral
- Each full turn of the helix has 3 Si and 3 O
atoms - Six helices combine to give a hexagonal structure
14
- Other Silicates also have SiO4 units in chains,
sheets, rings, arrays, etc - Al3 can substitute for Si4 Aluminosilicates
- Other cations needed to balance charge
- Al3, Mg2, Fe2, Ti3 common cations occupy
holes - Kaolinite Al2(OH)2Si4O10
- Talc 3 Mg substitute for 2 Al Mg3 (OH)2Si4O10
15
- Mica
- Layers of K ions between silicate and aluminate
layers - Al in about 25 of the Si positions
- Can be cleaved into incredibly smooth, flat
sheets - Used in nail polish
- Asbestos fibrous mineral
- Chrysotile Mg3(OH)4Si2O5
- Mg and Silicate layers differ in size leading to
curling fibrous structure - Zeolites mixed aluminosilicates with
- (Si,Al)nO2n frameworks and cations added to
balance the charge - Used as water softeners before polymer resins
developed (Cation exchange) - Cavities large enough for molecules to enter may
make stable complex - Water removal from organic solvent Molecular
Sieves - Cat litter and oil absorbant
- Catalysts and catalyst support for petroleum
cracking