1. Synthesis Reactions - PowerPoint PPT Presentation
1 / 13
Title:
1. Synthesis Reactions
Description:
Chapter 6: Chemical Reactions Occur in Predictable Ways 1. Synthesis Reactions Occur when two or more reactants (usually elements) join to form a compound. – PowerPoint PPT presentation
Number of Views:217
Avg rating:3.0/5.0
Title: 1. Synthesis Reactions
1
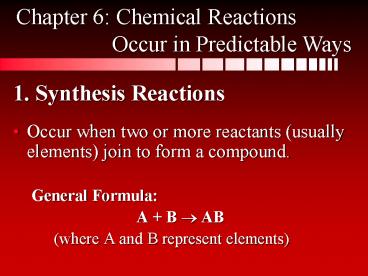
1. Synthesis Reactions
Chapter 6 Chemical Reactions Occur in
Predictable Ways
- Occur when two or more reactants (usually
elements) join to form a compound. - General Formula
- A B ? AB
- (where A and B represent elements)
2
can produce ionic or covalent compounds
- Ionic
- Magnesium metal reacts with oxygen
- gas to form magnesium oxide.
- 2Mg O2 ? 2MgO
- Covalent
- Nitrogen gas and oxygen gas join to form
dinitrogen monoxide. - 2N2 O2 ? 2N2O
3
2. Decomposition
- are the opposite of synthesis reactions
- A compound breaks down into two or more products
(often elements). - General Formula
- AB ? A B
- (where A and B represent elements)
4
Ionic and Covalent Compounds can Decompose
- Ionic
- Table salt, sodium chloride, can be broken down
into sodium metal and chlorine gas by melting
salt at 800ºC and running electricity through it. - 2NaCl ? 2Na Cl2
- Covalent
- By running electricity through water,
- the water molecules decompose into
- hydrogen and oxygen gases.
- 2H2O ? 2H2 O2
5
3. Single Replacement
- When one element from a compound is replaced with
a separate element - Two types, based on whether the single element is
a metal or a non-metal - General Forms
- A BC ? B AC where A is a metal, or
- A BC ? C BA where A is a non-metal
6
The Two Types
- When A is a metal
- Aluminum foil in a solution of copper II chloride
produces solid copper and aluminum chloride. - Al CuCl2 ? Cu AlCl3
- When A is a non-metal
- When fluorine is bubbled through a sodium iodide
solution, iodine and sodium fluoride are
produced. - F2 NaI ? I2 NaF
7
4. Double Replacement
- elements swap places between two compounds to
form two new compounds. - Two ionic solutions react to form a precipitate
(solid) and another ionic solution - General Form
- AB CD ? AD CB
8
4. Double Replacement Example
- potassium chromate and silver nitrate react to
form a red precipitate, silver chromate, in a
solution of potassium nitrate. - K2CrO4(aq) AgNO3(aq) ? Ag2CrO4(s) KNO3(aq)
Note the K and Ag switch places in the compounds.
9
5. Neutralization (aka Acid-Base reactions)
- occur when an acid and a base react to form a
salt and water. - acids (start with H)
- bases (end in OH, or begin with NH4)
- General Form
- Acid base ? salt water
- HX MOH ? MX H2O
- (where X and M are elements)
10
5. Neutralization Examples
- Sulphuric acid is used to neutralize calcium
hydroxide - H2SO4 Ca(OH) 2 ? CaSO4 2H2O
- Phosphoric acid helps to neutralize the compounds
that cause rust, such as iron (II) hydroxide. - 2H3PO4 3Fe(OH)2 ? Fe3(PO4)2 6H2O
11
6. Combustion
- occurs when a compound or element reacts with
oxygen - Always makes CO2 and H2O (in grade 10 examples)
- Aka. hydrocarbon combustion
- General Form
- CXHY O2 ? CO2 H2O
12
6. Combustion Examples
- Natural gas (methane) is burned in furnaces to
heat homes - CH4 O2 ? CO2 2H2O
- An acetylene torch is used to weld metals
together - 2C2H2 5O2 ? 4CO2 2H2O
- Carbohydrates like glucose combine with oxygen in
our body to release energy - C6H12O6 6O2 ? 6CO2 6H2O
Take the Section 6.1 Quiz
13
(No Transcript)