Chemistry Jeopardy - PowerPoint PPT Presentation
1 / 26
Title:
Chemistry Jeopardy
Description:
Title: Chemistry Jeopardy Author: Nicole M. Dunn Last modified by: BVASD Created Date: 5/22/2004 1:19:09 AM Document presentation format: On-screen Show (4:3) – PowerPoint PPT presentation
Number of Views:161
Avg rating:3.0/5.0
Title: Chemistry Jeopardy
1
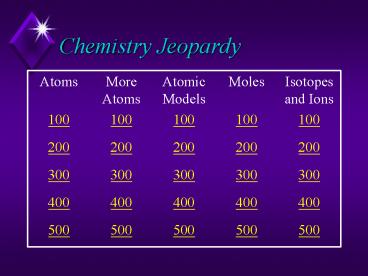
Chemistry Jeopardy
Atoms More Atoms Atomic Models Moles Isotopes and Ions
100 100 100 100 100
200 200 200 200 200
300 300 300 300 300
400 400 400 400 400
500 500 500 500 500
2
Atoms-100
- This element has 29 protons.
- What is copper (Cu)?
3
Atoms-200
- The mass number if an element of sulfur has 16
protons and 18 neutrons. - What is 34?
4
Atoms-300
- This part of the atom has a negative charge.
- What is the electron?
5
Atoms-400
- This element has an atomic mass of 79.
- What is selenium?
6
Atoms-500
- This element has an atomic number of 32.
- What is Germanium?
7
More Atoms-100
- The number of protons in rubidium.
- What is 37?
8
More Atoms-200
- The name of the element with 51 electrons.
- What is antimony?
9
More Atoms-300
- The number of neutrons in cesium.
- What is 78?
10
More Atoms-400
- The charge of sulfur if it has 18 electrons.
- What is 2?
11
More Atoms-500
- The number of electrons in strontium if it has a
charge of 2. - What is 36?
12
Atomic Models-100
- The name of the theory by J.J. Thompson.
- What is the plum-pudding model?
13
Atomic Models-200
- He discovered neutrons.
- Who is James Chadwick?
14
Atomic Models-300
- The scientist who conducted the Gold Foil
Experiment - Who is Rutherford?
15
Atomic Models-400
- The experiment Thomson performed
- What is the cathode ray tube experiment?
16
Atomic Models-500
- Name one part of Daltons atomic theory.
- What is?
17
Moles-100
- 3.000 moles Na ______ grams
- What is 68.97 grams?
18
Moles-200
- 810. g Cl _______ moles Cl
- What is 22.8 moles?
19
Moles-300
- 3.27 moles of Cl ____ atoms Cl
- What is 1.97 x 10 24 atoms?
20
Moles-400
- 7.7 x 1025 atoms Ni ____ g Ni
- What is 7.5 x 103 grams?
21
Moles-500
- 120. grams O ___ atoms
- What is 4.52 x 1024 atoms?
22
Isotopes and Ions-100
- Isotopes are different because they have
different_______. - What is neutrons or mass?
23
Isotopes and Ions-200
- The number of electrons in Al3.
- What is 10?
24
Isotopes and Ions-300
- The number of neutrons in uranium-235.
- What is 143?
25
Isotopes and Ions-400
- Compare and contrast carbon-14 and carbon-12.
- What is they have the same number of protons (6)
and electrons(6) and a different number of
neutrons (8 and 6 respectively)?
26
Isotopes and Ions-500
- The number of protons, neutrons, and electrons in
125I-1. - What is 53 protons, 72 neutrons, and 54 electrons?