International System of Units - PowerPoint PPT Presentation
1 / 23
Title:
International System of Units
Description:
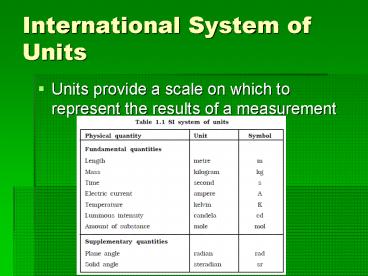
International System of Units Units provide a scale on which to represent the results of a measurement Measurements of Length Length - Fundamental unit is the meter 1 ... – PowerPoint PPT presentation
Number of Views:61
Avg rating:3.0/5.0
Title: International System of Units
1
International System of Units
- Units provide a scale on which to represent the
results of a measurement
2
(No Transcript)
3
Measurements of Length
- Length
- - Fundamental unit is the meter
- 1 meter 39.37 inches
4
Measurements of Volume
- Volume
- - amount of 3-D space occupies by a substance
- - fundamental unit is meter3 (m3)
- 1 cm3 1 ml
5
Items for measuring volume
- Graduated cylinder- useful for dispensing
approximate volumes - Buret- must be used when accuracy is important
- Volumetric flask- contains a specified volume of
liquid
6
(No Transcript)
7
Measuring Mass
- Weight- mass times gravity
- Mass- the amount of matter in an object
- - quantity of matter in an object
- - fundamental unit is the kilogram
8
Measuring Density
- Density mass/ volume
- Derived unit- a unit obtained from more than one
base unit. - Density- is a characteristic property that
depends only on the composition of a substance,
not on the size of the sample. - Pure substances have specific densities.
- The density of a substance generally decreases as
its temperature increases. Water is an important
exception
9
(No Transcript)
10
Density Problems
- What is the density of a block of marble that
occupies 310cm3 and has a mass of 853g? - Diamond has a density of 3.26g/cm3. What is the
mass of a diamond that has a volume of 0.350cm3? - What is the volume of a sample of liquid mercury
that has a mass of 76.2g, given that the density
of mercury is 13.6g/ml?
11
Measuring Specific Gravity
- Specific Gravity- a comparison of the density of
a reference substance. Water is a common
reference substance. - Can be determined with a hydrometer
- Specific Gravity density of substance/ density
of water - - Has no units
12
Measuring Temperature
- Temperature- is related to the energy and motion
of particles - Temperature scales
- Celsius scale- based on the freezing and
boiling points of water. - a. freezing point 0 C
- b. boiling point 100 C
13
- Kelvin scale- The zero point on the Kelvin scale
is absolute zero. The theoretical point at which
all motion stops. One degree Kelvin equals one
degree celsius - K C 273
- C K - 273
14
(No Transcript)
15
Measuring Temperature
- Practice Problems
- Normal human body temperature is 37 C. What is
your temperature in Kelvin? - The boiling point of the element Argon is 87 K.
What is the boiling point of argon in C?
16
The Importance of measurement
- Scientific Notation
17
Scientific notation
- 36,000 is 3.6 x 104
- The exponent indicates how many times the
coefficient 3.6 must be multiplied by 10 to equal
the number 36,000. - Large numbers have positive exponents
18
- Small numbers (less than one) have negative
exponents. - 0.0081 is 8.1 x 10-3, the exponent -3 indicates
that the coefficient 8.1 must be divided by 10
three times to equal 0.0081
19
Multiplication and Division
- To multiply numbers written in scientific
notation, multiply the coefficients and add the
exponents - (3.0 x 104) x (2.0 x 102) 6.0 x 106
20
- To divide numbers written in scientific notation,
first divide the coefficient, then subtract the
exponent in the dominator from the exponent in
the numerator - 3.0 x 104 / 2.0 x 102 1.5 x 102
21
Sample Problems
- Write each measurement in scientific notation
- 222 meters
- 2.22 x 102 meters
- 728500 meters
- 7.285 x 105 meters
- 0.00054 meters
- 5.4 x 10-4 meters
22
- Multiplication and Division
- (3.0 x 105) x (2.6 x 103)
- 7.8 x 108
- (2.8 x 103) / (1.4 x 10-2)
- 2.0 x 105
23
Conversion Factors
- Conversion factor- a ration of eqvivalent
measurements, such as 100cm / 1m - The measurement in the numerator is equivalent to
the measurement in the denominator. - Conversion factors are exact quantities.
Therefore, they have an unlimited number of
significant figures.