Spectral Tuning in Retinal Proteins - PowerPoint PPT Presentation
Title:
Spectral Tuning in Retinal Proteins
Description:
Spectral Tuning in Retinal Proteins hn all-trans 11-cis – PowerPoint PPT presentation
Number of Views:128
Avg rating:3.0/5.0
Title: Spectral Tuning in Retinal Proteins
1
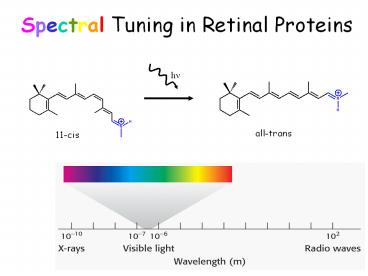
Spectral Tuning in Retinal Proteins
all-trans
11-cis
2
Color Vision
all-trans
11-cis
3
Visual Receptors
Light
G-protein signaling pathway
Rhodopsin
4
Spectral tuning in color visual receptors
Color is sensed by red, green and blue rhodopsin
visual receptors.
500nm
600nm
400nm
absorption spectrum
Their chromophores are exactly the same!
11-cis
How does the protein tune its absorption spectrum?
5
Spectral Tuning in bacteriorhodpsins photocycle
6
How can we change the maximal absorption of
retinal chromophore?
7
Excitation energy determines the maximal
absorption
S1
S0
Response
13
7
9
11
15
8
Electronic Absorption
p-p
Absorption of light in the UV-VIS region of the
spectrum is due to excitation of electrons to
higher energy levels.
9
p-p excitation in polyenes
p
p
DE
E
photon
p
p
Ground state (S0)
Excited state (S1)
DE (excitation energy, band gap) hn hc/l
10
p-p excitation in polyenes
p
p
p
E
p
p
p
blue-shift
red-shift
11
p-p excitation in polyenes
12
Tuning the length of the conjugated backbone
b-carotene
Vitamin A1 (retinal I)
Vitamin A2 (retinal II)
Longer wavelength
Short wavelength
13
Retinal II
Retinal I
Salmon different retinals in different stages of
life
14
OPSIN SHIFT how protein tunes the absorption
maximum of its chromophore.
Maximal absorption of protonated retinal Schiff
base in Water/methanol solution 440 nm bR
568 nm rod Rh 500 nm red receptor 560
nm green receptor 530 nm blue receptor 426 nm
15
Electrostatics and opsin shift
S2
positive charge
S2
S1
Me
Me
Me
S1
N
S0
H
O
Me
O
Me
C
Asp (Glu)
S0
no protein
in protein
counterion
- The counterion stabilizes the positive charge of
the chromophore. - The position of the counterion determines how and
how much the band gap energy changes.
16
Electrostatics and opsin shift
S2
positive charge
S2
S1
Me
Me
Me
S1
N
S0
H
O
Me
O
Me
C
Asp (Glu)
S0
no protein
in protein
counterion
Maximal absopriton of protonated retinal Schiff
base can be changed by D85N (Purple to blue
shift) Red shift from 568 to 605 nm at pH 3
17
Dinosaurs had red-shifted visual receptors!
Howard Hughes Medical Institute at The
Rockefeller University and Yale University
18
Microbial rhodopsins in Halobacteria
Bacteriorhodopsin (bR) proton
pump Halorhodopsin (hR) chloride pump
purple membrane
Sensory rhodopsin I (sRI) attractant (repellent)
to orange (near UV) light Sensory rhodopsin II
(sRII) repellent to blue-green
light phototaxis (color vision of halobacteria)
hR
sRII
sRI
bR
500nm
600nm
19
Spectral Tuning in Bacterial Rhodopsins
Sensory Rhodopsin II (sRII)
Phototaxis
Bacteriorhodopsin (bR) Proton pump
- Large blue shift of absorption maximum in sRII
(70 nm)
sRII
bR
500nm
600nm
20
Structures of bR and sRII
X-ray crystallography shows that structures are
very similar. Both include protonated all-trans
retinal Schiff base
orange sRII purple bR
21
Binding Sites of bR and sRII
- Similar structure
- Aromatic residues.
- Hydrogen-bond network.
- (counter-ion asparatates,
- internal water molecules)
Mutagenic substitutions
T204A/V108M/G130S of sRII produces only 20 nm
(30) spectral shift.
What is the main determinant(s) of spectral
tuning?
22
QM/MM Calculation of spectral shift in bR and
sR-II
QM/MM
helix G
- Refinement of X-ray structures by HF (retinal,
2Asp, 3H2O) - Excitation energy calculations for retinal
retinal-K205/216
D201/212
bR purple, sRII orange
Calculated spectra
DE(S1-S0)
Spectral shift
sRII
bR
DE(S1-S0) 6.1 (exp. 7.2) kcal/mol DE(S2-S0 )
1.7 (exp. 4.0) kcal/mol
A sub-band in sRII is due to the second excited
state (S2).
500nm
600nm
23
Deprotonation of the Schiff base
UV vision birds, honeybee
Strong blue shift
24
p atomic orbitals
Planarity is essential for maximal overlap of p
orbitals in a double bond (p molecular orbital)
25
Steric interactions and spectral shift?
A highly twisted structure can decrease the
overlap of p orbitals and effectively decrease
the length of the conjugation, i.e., blue shift.
26
Summary of Mechanisms of Spectral Tuning
- Using a different chromophore with a longer or a
shorter conjugated chain - Modifying the amino acid composition of the
binding pocket (electrostatics) - Manipulating the distance and/or conformation of
charged/polar groups in the vicinity of retinal - Steric interaction with the chromophore so that
some of the double bonds go out of plane (a
similar effect to using a shorter chromophore) - Protonation state of retinal Schiff base (Strong
blue shift upon deprotonation)
27
The chromophore retinal adopts different colors
in different environments. Doesnt it remind you
of something?