Matter, Solutions, and Gas Laws - PowerPoint PPT Presentation
Title:
Matter, Solutions, and Gas Laws
Description:
Matter, Solutions, and Gas Laws – PowerPoint PPT presentation
Number of Views:94
Avg rating:3.0/5.0
Title: Matter, Solutions, and Gas Laws
1
Matter, Solutions, and Gas Laws
2
Georgia Performance Standards
- SPS2. Students will explore the nature of matter,
its classifications, and its system for naming
types of matter. - a. Calculate density when given a means to
determine a substances mass and volume. - SPS5a. Compare and contrast the atomic/molecular
motion of solids, liquids, gases and plasmas. - SPS7d. Explain the flow of energy in phase
changes through the use of a phase diagram.
3
Properties of Matter
- Physical Property- a characteristic of a material
that you can observe without changing its
identity. - Ex.- Color, ____________, __________, magnetic
quality, malleability, ability to flow, odor,
state of matter, density, melting point
4
Density
- The amount of mass per unit volume
- Density mass/volume
- Explain why the density of an object does not
change if you cut it into smaller pieces. IF YOU
CUT A PIECE OF STEEL IN HALF, THE MASS IS CUT IN
HALF, AND THE VOLUME IS CUT IN HALF - Example 20 grams of steel cut in half is 10
grams (mass) and the volume is cut in half from 5
cubic centimeters to 2.5 cubic centimeters. - mass/volume mass/ volume
- 20g / 5 cm3 4 g/ cm3 or 10/ 2.5 cm3
4 g/ cm3 - SAME DENSITY!!!
- DENSITY OF AN MATERIAL NEVER CHANGES... NO MATTER
HOW BIG OR SMALL!!
5
What are the physical properties of these items?
6
- During a Physical Change the internal makeup of a
substance ____________ change. - Ex.- Freezing, Melting, Boiling, condensing,
cutting into , distillation.
7
Distillation
- physical change
- The process of
- separating a mixture
- by its boiling points
- Examples
- Making alcohol, separating petroleum, or salt
water
8
Petroleum Fractional Distillation
Oil was formed from the remains of animals and
plants that lived millions of years ago. Over
the years, the remains were covered by layers of
mud. Heat and pressure from these layers helped
the remains turn into what we today call crude
oil . The word "petroleum" means "rock oil" or
"oil from the earth."
Other products made from petroleum include ink,
crayons, bubble gum, dishwashing liquids,
deodorant, eyeglasses, records, tires, ammonia,
and heart valves.
9
Other materials made from petroleum
- Solvents Diesel Motor Oil Bearing Grease Ink
Floor Wax Ballpoint Pens Football Cleats
Upholstery Sweaters Boats Insecticides Bicycle
Tires Sports Car Bodies Nail Polish Fishing lures
Dresses Tires Golf Bags Perfumes Cassettes
Dishwasher Tool Boxes Shoe Polish Motorcycle
Helmet Caulking Petroleum Jelly Transparent Tape
CD Player Faucet Washers Antiseptics Clothesline
Curtains Food Preservatives Basketballs Soap
Vitamin Capsules Antihistamines Purses Shoes
Dashboards Cortisone Deodorant Footballs Putty
Dyes Panty Hose Refrigerant Percolators Life
Jackets Rubbing Alcohol Linings Skis TV Cabinets
Shag Rugs Electrician's Tape Tool Racks Car
Battery Cases Epoxy Paint Mops Slacks Insect
Repellent Oil Filters Umbrellas Yarn Fertilizers
Hair Coloring Roofing Toilet Seats Fishing Rods
Lipstick Denture Adhesive Linoleum Ice Cube Trays
Synthetic Rubber Speakers Plastic Wood Electric
Blankets Glycerin Tennis Rackets Rubber Cement
Fishing Boots Dice Nylon Rope Candles Trash Bags
House Paint Water Pipes Hand Lotion Roller Skates
Surf Boards Shampoo Wheels Paint Rollers Shower
Curtains Guitar Strings Luggage Aspirin Safety
Glasses Antifreeze Football Helmets Awnings
Eyeglasses Clothes Toothbrushes Ice Chests
Footballs Combs CD's Paint Brushes Detergents
Vaporizers Balloons Sun Glasses Tents Heart
Valves Crayons Parachutes Telephones Enamel
Pillows Dishes Cameras Anesthetics Artificial
Turf Artificial limbs Bandages Dentures Model
Cars Folding Doors Hair Curlers Cold cream Movie
film Soft Contact lenses Drinking Cups Fan Belts
Car Enamel Shaving Cream Ammonia Refrigerators
Golf Balls Toothpaste Gasoline
10
Properties of Matter
- Chemical Property-- describes its "potential" to
undergo some chemical change or reaction because
of its composition - Chemical properties can only be observed by
changing a substance's identity. Flammability,
Reactivity, etc - Once a chemical change has occurred a NEW
SUBSTANCE OR SUBSTANCES is/are produced with
totally new physical and chemical
characteristics.
11
Examples of Chemical Changes
12
Chemical Changes
- Soft, silver metal
- Reacts violently
- with water
- Green gas
- toxic
- Love it on French
- fries
- Need it to live
13
Conservation of Mass
- During a chemical reaction, energy is taken in or
given off - The Law of Conservation of Mass states mass is
neither created or destroyed. - The mass of the reactant(s) is equal to the mass
of the products(s).
reactants
products
14
Composition of MatterPure Substances and Mixture
- Pure Substances Elements and Compounds
- A. Elements all atoms in the substance are
alike - 90 elements found in nature
- 20 made in laboratories
- Atom is the smallest particle of an element that
still retains the characteristics of that element - Examples copper, gold, hydrogen, carbon
- (anything on the periodic table)
15
- Compounds consisting of two or more elements
bonded together (chemically combined) in a fixed
mass ratio that can be split into simpler
substances. - Examples water (H20), carbon dioxide (CO2),
sugar (C6H1206), hydrochloric acid (HCl), - salt (NaCl)
- Molecule -- the smallest particle of a compound
that still retains the characteristics of that
compound
16
- Mixtures
- 2 or more materials mixed together BUT NOT
CHEMICALLY combined, they still retain their own
chemical makeup. - Unlike compounds, mixtures do not always
- contain substances in fixed proportions.
17
Heterogeneous mixtures
18
Heterogeneous Mixtures
- 2. Suspension-- mixture between liquids or
liquids/solids that will settle out upon
standing. - Examples Italian dressing, pond water, oil and
vinegar
19
Homogeneous Mixtures
- 1. Solution-- A mixture where one material is
_______________ in another - the dissolved particles are so small you cant
see them
- Parts of a solution
- A. ___________ the particles dissolved
- in the solution
- Examples sugar, Koolaid mix, salt
- B. __________ the substance in a
- solution in which the particles
- dissolve
- Usually water
20
An Alloy is a Solution
- Alloy-- a solution of two or more elements,
usually metal and metal. - brass zinc and copper
- stainless steel copper, nickel and iron
- Pewter lead, copper, tin
- White gold nickel, palladium and gold
- Rose gold copper and gold
- Bronze aluminum and copper
What are 3 reasons we make alloys?
21
Homogeneous Mixture
- 2. Colloid A homogeneous mixture that contains
some particles that are larger in size, but still
evenly distributed throughout - Does not settle upon standing
- Tyndall Effect scattering of light due to larger
particles causes milky/cloudy color in colloids - Example milk, fog, peanut butter, butter,
mayonnaise, yogurt
22
Gas Laws
- Charles Law
- STATESAs ___________ goes up, __________ goes up
(if pressure stays same) - Remember CTV
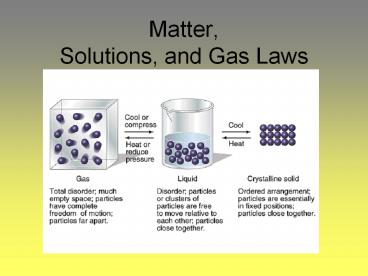
- Volume is directly proportional to temperature
- Example basketball in the summer vs. basketball
in the winter
23
Gas Laws
- Boyles Law
- STATES..If the ___________ goes down, then the
___________ will go up (if the temperature stays
constant) - Remember BVP
- Pressure is indirectly proportional to volume
Larger volume, less pressure
smaller volume, pressure goes up