Drill: Draw LDDs for: - PowerPoint PPT Presentation
1 / 111
Title:
Drill: Draw LDDs for:
Description:
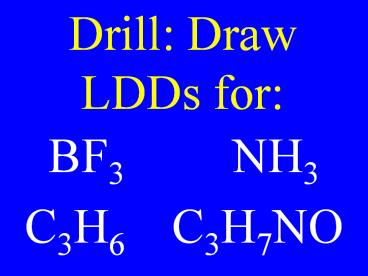
Drill: Draw LDDs for: BF3 NH3 C3H6 C3H7NO – PowerPoint PPT presentation
Number of Views:98
Avg rating:3.0/5.0
Title: Drill: Draw LDDs for:
1
Drill Draw LDDs for
- BF3 NH3
- C3H6 C3H7NO
2
Solutions
3
Solution
- Homogeneous mixture made up of at least one
solute dissolved in the solvent
4
Solute
- Substance being dissolved
- Portion in lesser molar amount
5
Solvent
- Substance doing the dissolving
- Portion in greatest molar amount
6
Colloid
- Slightly larger particles
- Light passes particles stay suspended
7
Suspension
- Even larger particles
- Particles block or reflect light
8
Tyndall Effect
- Because light reflects off suspended particles,
the light ray can be seen from the side
9
Size Comparison
- Solution lt Colloid
- Colloid lt Suspension
10
Soluble
- When one substance (solute) dissolves in another
(solvent)
11
Solubility
- The max amount of one substance (solute)
dissolved in another (solvent)
12
Concentration
- The amount of solute dissolved into solution
13
ConcentratedSolution
- A solution with a relatively large amount of
solute dissolved
14
Dilute Solution
- A solution with a relatively small amount of
solute dissolved
15
Saturated Solution
- A solution with the maximum amount of solute
dissolved in the solution
16
Unsaturated Solution
- A solution with less than the maximum amount of
solute dissolved in solution
17
Supersaturated Solution
- A solution with greater than the maximum amount
of solute dissolved in solution
18
Drill Draw LDDs for
C4H8 HNO3 C4H6O
19
Solution Chemistry
20
Solution Measures
- Concentration soln
- Molarity
- Molality
- Mole Fraction
21
Percent Solution
- Mass of one portion per the total mass, all times
100 - soln ma/mtotal x 100
22
Molarity
- Moles of solute per liter of solution
- M molessolute/Lsoln
23
Molality
- Moles of solute per kilogram of solvent
- mo molessolute/kgsolvent
24
Mole Fraction
- Moles of one portion per total number of moles in
the solution - X molesa/molessoln
25
Calculate the molarity of a 250 mL solution
containing 5.0 g NaOH dissolved in water
26
Calculate the molality of 69 g of C2H5OH
dissolved in 500.0 mL of water
27
Calculate the mole fraction of each portion when
92 g of C2H5OH dissolved in 144 mL of water
28
Calculate the molality mole fraction of a
solution containing 46 g of C2H5OH dissolved in
1782 mL of water
29
Drill
- Calculate the mass of KI required to make 250 mL
of 0.500 M KI.
30
Colligative Properties
- Properties dependent only on the concentration of
particles in solution
31
Examples
- Vapor pressure
- Boiling Freezing points
- Osmotic pressure
32
(No Transcript)
33
Vapor Pressure
- VPsolution (VPsolvent)(Xsolvent)
- X mole fraction
- VP vapor pressure
34
Boiling Freezing
DT imoK DT change in BP or FP i ionic
activity K BP or FP constant
35
Osmotic Pressure
p iMRT p osmotic pressure i ionic
activity M Molarity
36
Calculate the vapor pressure of a solution
containing 150 g C5H10O5 in 162 mL of water at
30oC
37
Calculate BP FP of 60.0 g of NaOH in 250 mL
waterKBP 0.512oC/moKFP -1.86oC/mo
38
Calculate the osmotic pressure of a solution
containing 12 g of NaOH dissolved in 250 mL
solution at 27oC
39
Drill
- Calculate the VP of a solution containing 36
m/m glucose (C6H12O6) in water at 29oC - (VPwater 30.0 mm Hg)
40
Drill Draw LDDs for
- BF3 NH3
- C3H6 C3H7NO
41
Are there any questions on previous material?
42
Solutions
43
Solution
- Homogeneous mixture made up of at least one
solute dissolved in the solvent
44
Solute
- Substance being dissolved
- Portion in lesser molar amount
45
Solvent
- Substance doing the dissolving
- Portion in greatest molar amount
46
Colloid
- Slightly larger particles
- Light passes particles stay suspended
47
Suspension
- Even larger particles
- Particles block or reflect light
48
Tyndall Effect
- Because light reflects off suspended particles,
the light ray can be seen from the side
49
Size Comparison
- Solution lt Colloid
- Colloid lt Suspension
50
Soluble
- When one substance (solute) dissolves in another
(solvent)
51
Solubility
- The max amount of one substance (solute)
dissolved in another (solvent)
52
Concentration
- The amount of solute dissolved into solution
53
ConcentratedSolution
- A solution with a relatively large amount of
solute dissolved
54
Dilute Solution
- A solution with a relatively small amount of
solute dissolved
55
Saturated Solution
- A solution with the maximum amount of solute
dissolved in the solution
56
Unsaturated Solution
- A solution with less than the maximum amount of
solute dissolved in solution
57
Supersaturated Solution
- A solution with greater than the maximum amount
of solute dissolved in solution
58
Drill Draw LDDs for
C4H8 HNO3 C4H6O
59
Solution Chemistry
60
Solution Measures
- Concentration soln
- Molarity
- Molality
- Mole Fraction
61
Percent Solution
- Mass of one portion per the total mass, all times
100 - soln ma/mtotal x 100
62
Molarity
- Moles of solute per liter of solution
- M molessolute/Lsoln
63
Molality
- Moles of solute per kilogram of solvent
- mo molessolute/kgsolvent
64
Mole Fraction
- Moles of one portion per total number of moles in
the solution - X molesa/molessoln
65
Calculate the molarity of a 250 mL solution
containing 5.0 g NaOH dissolved in water
66
Calculate the molality of 69 g of C2H5OH
dissolved in 500.0 mL of water
67
Calculate the mole fraction of each portion when
92 g of C2H5OH dissolved in 144 mL of water
68
Calculate the molality mole fraction of a
solution containing 46 g of C2H5OH dissolved in
1782 mL of water
69
Drill
- Calculate the mass of KI required to make 250 mL
of 0.500 M KI.
70
Are there any questions on previous material?
71
Colligative Properties
- Properties dependent only on the concentration of
particles in solution
72
Examples
- Vapor pressure
- Boiling Freezing points
- Osmotic pressure
73
(No Transcript)
74
Vapor Pressure
- VPsolution (VPsolvent)(Xsolvent)
- X mole fraction
- VP vapor pressure
75
Boiling Freezing
DT imoK DT change in BP or FP i ionic
activity K BP or FP constant
76
Osmotic Pressure
p iMRT p osmotic pressure i ionic
activity M Molarity
77
Calculate the vapor pressure of a solution
containing 150 g C5H10O5 in 162 mL of water at
30oC
78
Calculate BP FP of 60.0 g of NaOH in 250 mL
waterKBP 0.512oC/moKFP -1.86oC/mo
79
Calculate the osmotic pressure of a solution
containing 12 g of NaOH dissolved in 250 mL
solution at 27oC
80
Drill
- Calculate the VP of a solution containing 36.0
m/m glucose (C6H12O6) in water at 29oC - (VPwater 30.0 mm Hg)
81
Test Review
82
Classify the type of Bonding
- Fe-Cl Fe-Cr
- C-Cl H-Br
- S-O K-I
83
Classify the type of IM Force
H2 Cl2 HF HF H2 HBr HBr HI CH4 C2H6
84
Draw LDDs for
- BH3 NCl3
- CF4 C3H6
- IF3 CO3-2
85
Calculate the BP of a solution containing 120 g
C3H7OH in 250 mL of water at its BP. KBP
0.512oC/mo KFP -1.86oC/mo
86
Calculate the osmotic pressure of a solution
containing 12 gof NaOH dissolved in 50.0 mL
solution at 27oC
87
Calculate the vapor pressure of a solution
containing 12.0 g C3H8O in 14.4 mL of water at
its BP. VPsolvent 120 kPa
88
Calculate the molarity of 33.1 g of Pb(NO3)2
dissolved in 250 mL of solution.
89
Draw LDDs for
PCl3 C3H7NO NO3-1
90
Calculate the mass of lead(II)nitrate required to
make 250 mL of 0.40 M Pb(NO3)2
91
Calculate the BP of a solution containing 29.9 g
of CoBr3 dissolved in 75 mL of water. KBP
0.512oC/mo
92
Calculate the molality of a solution that is 33.1
by mass Pb(NO3)2 in water
93
Drill Calculate the osmotic pressure of 0.88 g
of CO2 in 750 mL of soln at 27oC
94
30.0 g C3H6NF was dissolved in 50.0 g C6H12O (VP
20.0 kPa) at 27oC making a 0.800 g/mL solution.
Calculate X, mo, M, p, VP.
95
180 g C3H8O was dissolved in 180 mL H2O at 27oC
making a 1.5 g/mL solution. Calculate X, mo, M,
p, VP, BP, FP.
96
A 1.2 g/cm3 aqueous solution is 20.0 by mass
NaOH at 27oC. Calculate X, mo, M, p, MP
97
Calculate the molecular mass of a covalent
compound dissolved in an aqueous solution to make
it 25 by mass when it boils at 102.048oC
98
Are there any questions on previous material?
99
Test Review
100
Classify the type of Bonding
- Fe-Cl Fe-Cr
- C-Cl H-Br
- S-O K-I
101
Classify the type of IM Force
H2 Cl2 HF HF H2 HCl HBr HI CH4 C2H6
102
Draw LDDs for
- BH3 NCl3
- CF4 C3H6
- IF3 CO3-2
103
Calculate the FP BP of a solution containing
120 g C3H7OH in 250 mL of water at its BP. KBP
0.512oC/mo KFP -1.86oC/mo
104
Drill Calculate the molarity of 33.1 g of
Pb(NO3)2 dissolved in 250 mL of solution.
105
Calculate the BP of a solution containing 29.9 g
of CoBr3 dissolved in 75 mL of water. KBP
0.512oC/mo
106
Calculate the molality of a solution that is 33.1
by mass Pb(NO3)2 in water
107
Drill Calculate the osmotic pressure of 0.88 g
of CO2 in 750 mL of soln at 27oC
108
30.0 g C3H6NF was dissolved in 50.0 g C6H12O (VP
20.0 kPa) at 27oC making a 0.800 g/mL solution.
Calculate X, mo, M, p, VP.
109
180 g C3H8O was dissolved in 180 mL H2O at 27oC
making a 1.5 g/mL solution. Calculate X, mo, M,
p, VP, BP, FP.
110
A 1.2 g/cm3 aqueous solution is 20.0 by mass
NaOH at 27oC. Calculate X, mo, M, p, MP
111
Calculate the molecular mass of a covalent
compound dissolved in an aqueous solution to make
it 25 by mass when it boils at 102.048oC