The Development of Atomic Models - PowerPoint PPT Presentation
1 / 4
Title:
The Development of Atomic Models
Description:
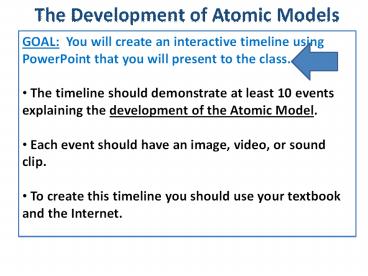
The Development of Atomic Models GOAL: You will create an interactive timeline using PowerPoint that you will present to the class. The timeline should demonstrate at ... – PowerPoint PPT presentation
Number of Views:80
Avg rating:3.0/5.0
Title: The Development of Atomic Models
1
The Development of Atomic Models
- GOAL You will create an interactive timeline
using PowerPoint that you will present to the
class. - The timeline should demonstrate at least 10
events explaining the development of the Atomic
Model. - Each event should have an image, video, or sound
clip. - To create this timeline you should use your
textbook and the Internet.
2
The Development of Atomic Models Example
1897
1803
John Dalton pictures atoms as tiny,
indestructible particles, with no internal
structure.
J.J. Thomson, a British scientist, discovers the
electron. The later leads to his "plumpudding
model. He pictures electrons embedded in a sphere
of positive electrical charge.
3
Bohr Model In Niels Bohr's model, the electrons
move in circular orbits at fixed distances from
the nucleus.
1913
1911
New Zealand physicist Ernest Rutherford finds
that an atom has a small, dense, positively
charged nucleus. Electrons move around the
nucleus.
4
Electron Cloud Model Erwin Schrödinger develops
mathematical equations to describe the motion of
electrons in atoms. His work leads to the
electron cloud model.
1926