Neutralization Reactions - PowerPoint PPT Presentation
Title:
Neutralization Reactions
Description:
Neutralization Reactions Ex: ... To determine the concentration of an acid or base through a neutralization reaction. Acid/Base Titration Buret is filled with a ... – PowerPoint PPT presentation
Number of Views:196
Avg rating:3.0/5.0
Title: Neutralization Reactions
1
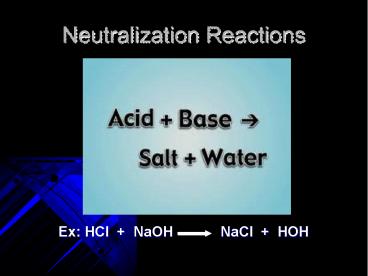
Neutralization Reactions
- Ex HCl NaOH NaCl HOH
2
- These are double replacement reactions
- Ions switch partners. Water is formed.
http//www.youtube.com/watch?v8IRI5gPR5EYsafeac
tive
3
Neutralization Forms Water
- H OH- ?? H2O
- from acid from base neutral
- This net reaction for neutralization is found
- on Table I. It is an exothermic reaction.
4
Forming a Salt
- () cation from the base
- (-) anion from the acid
- combine to form a salt.
5
- If equal concentrations of hydrogen ion H and
hydroxide ions OH- are mixed, it results in a
neutral solution. - Ex
- .1M LiOH .1M HCl Neutral
Solution - pH 7
6
Completing and Balancing Neutralization Equations
- Write charges for ions in acid and base.
- Switch ion partners. ( ions stay in front!!)
- Do not bring over subscripts except if part of a
polyatomic ion! - Criss-Cross charges to balance formulas.
- Balance entire equation.
- Name the acid, base and salt.
7
You Try It
- Complete equation and balance formulas
- Balance equation.
- Name acid base and salt
- Ex KOH H2SO4
- KOH H2SO4 HOH K2SO4
- Ex Mg(OH)2 HNO3
- Mg(OH)2 HNO3 HOH Mg(NO3)2
8
Hydrolysis
- Opposite reaction to neutralization
- Salt Water Acid Base
9
Parent Acid/Base
- If you know the salt involved you should be able
to determine which acid and base it would form if
water is added. - Salt Water Acid Base
- Ex
- NaCl with water (HOH) would form HCl and NaOH
10
You Try It
- Name the parent acid and base that would be
produced from these salts. - Ex Potassium chloride
- Magnesium carbonate
11
Lets Practice
- Which substance is always a product when an
Arrhenius acid in an aqueous solution reacts with
an Arrhenius base in an aqueous solution? - (1) HBr
- (2) KBr
- (3) H2O
- (4) KOH
12
- Which word equation represents a neutralization
reaction? - (1) base acid ? salt water
- (2) base salt ? water acid
- (3) salt acid ? base water
- (4) salt water ? acid base
13
- What are the products of a reaction between
KOH(aq) and HCl(aq)? - (1) H2 and KClO
- (2) KH and HClO
- (3) H2O and KCl
- (4) KOH and HCl
14
- Sulfuric acid, H2SO4(aq), can be used to
neutralize barium hydroxide, Ba(OH)2(aq). What
is the formula for the salt produced by this
neutralization? - (1) BaS
- (3) BaSO3
- (2) BaSO2
- (4) BaSO4
15
- Which compound could serve as a reactant in a
neutralization reaction? - (1) NaCl
- (2) CH3OH
- (3) KOH
- (4) CH3CHO
16
- Answer 3
17
Titrations
- Purpose
- To determine the concentration of an acid or
base through a neutralization reaction.
18
Acid/Base Titration
- Buret is filled with a
- standard solution of
- known concentration (M).
- Erlenmeyer Flask contains
- solution of unknown conc..
- Indicator (phenolphthalein)
- added to the flask.
19
Acid/Base Titrations
- Slowly titrate or drip liquid
- into flask from buret until
- indicator changes color.
- This is the endpoint or
- equivalence point.
- (Moles H Moles OH-)
- Record total volume (V) used
- from buret.
20
Titration Formula
- MA x VA MB x VB
- Molarity Acid x Volume Acid Molarity Base x
Volume Base
21
- Titration Applet
- http//group.chem.iastate.edu/Greenbowe/sections/p
rojectfolder/flashfiles/stoichiometry/acid_base.ht
ml
22
You Try It!
- A 50 ml sample of .2M HCl is neutralized by
- 75ml of NaOH. What is the conc. of the base?
- HCl NaOH
- .2M x 50ml MB x 75ml
- MB .13M
23
Important Note
- Multiply acid/base side of equation by number of
H or OH- ions it produces when dissociating!!! - Ex
- H2SO4 produces 2 H so you would multiply the
acid side by 2
24
You Try It!
- How much of a .1M H2SO4 solution is
- needed to neutralize 50 ml of a .05 KOH
- solution?
- H2SO4 KOH
- 2 x .1M x VA .05M x 50ml
- VA 12.5 ml
25
- Titration Demonstration
- http//www.youtube.com/watch?v5BZ0MPIgeEEsafeac
tive
26
Lets Practice
- During which process can 10.0 milliliters of a
0.05 M HCl(aq) solution be used to determine the
unknown concentration of a given volume of
NaOH(aq) solution? - (1) evaporation
- (2) filtration
- (3) distillation
- (4) titration
27
- The data collected from a laboratory titration
are used to calculate the - (1) rate of a chemical reaction
- (2) heat of a chemical reaction
- (3) concentration of a solution
- (4) boiling point of a solution
28
- A student completes a titration by adding
- 12.0 ml of NaOH(aq) of unknown concentration
- to 16.0 ml of 0.15 M HCl(aq).
- What is the molar concentration of the NaOH(aq)?
- (1) 0.11 M
- (2) 1.1 M
- (3) 0.20 M
- (4) 5.0 M